Preparation method and application of diphenolic acid based nonisocyanate polyurethane
A non-isocyanate, bisphenolic acid isopropyl alcohol ester technology, applied in the preparation of bisphenolic acid-based non-isocyanate polyurethane, the application field of preparing bisphenolic acid-based non-isocyanate polyurethane composite coating, can solve high toxicity and high pollution , Polyurethane does not meet the requirements of green development, etc., to achieve high regularity, strength, and excellent mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] The preparation of step 1 bisphenolic acid isopropyl alcohol ester
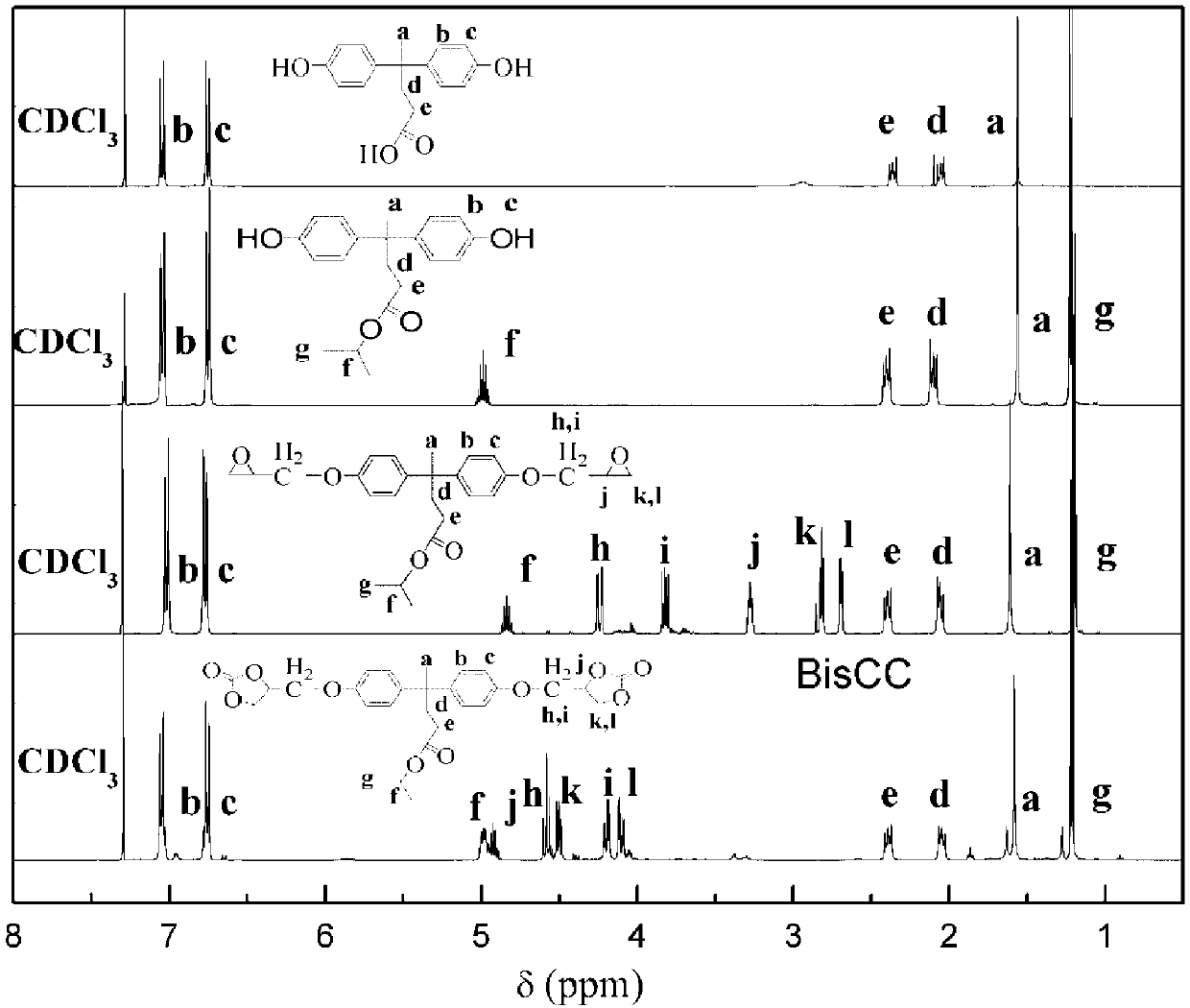
[0080] Add 50g of bisphenolic acid, 400mL of isopropanol, and 1ml of concentrated sulfuric acid into a 1L single-necked flask, stir and raise the temperature to reflux (~95°C). After 24 hours of reaction, most of the solvent is removed by rotary evaporation. The crude product was dissolved in 250 mL of ethyl acetate, washed twice with 10%wt sodium bicarbonate solution, and then washed with deionized water until the pH of the aqueous layer was 7. After removing excess moisture in the organic layer with anhydrous magnesium sulfate, the ethyl acetate was removed by rotary evaporation and the product was dried in a vacuum oven to obtain 49.80 g of light yellow solid isopropanol bisphenolate. (Yield: 87%)
[0081] The preparation of step 2 bisphenolic acid isopropyl alcohol ester diglycidyl ether
[0082] Add 25g isopropanol bisphenolate, 121g epichlorohydrin (15eqiuv.), 115mL isopropanol in a 1L three-ne...
Embodiment 2
[0090] Step 1~3
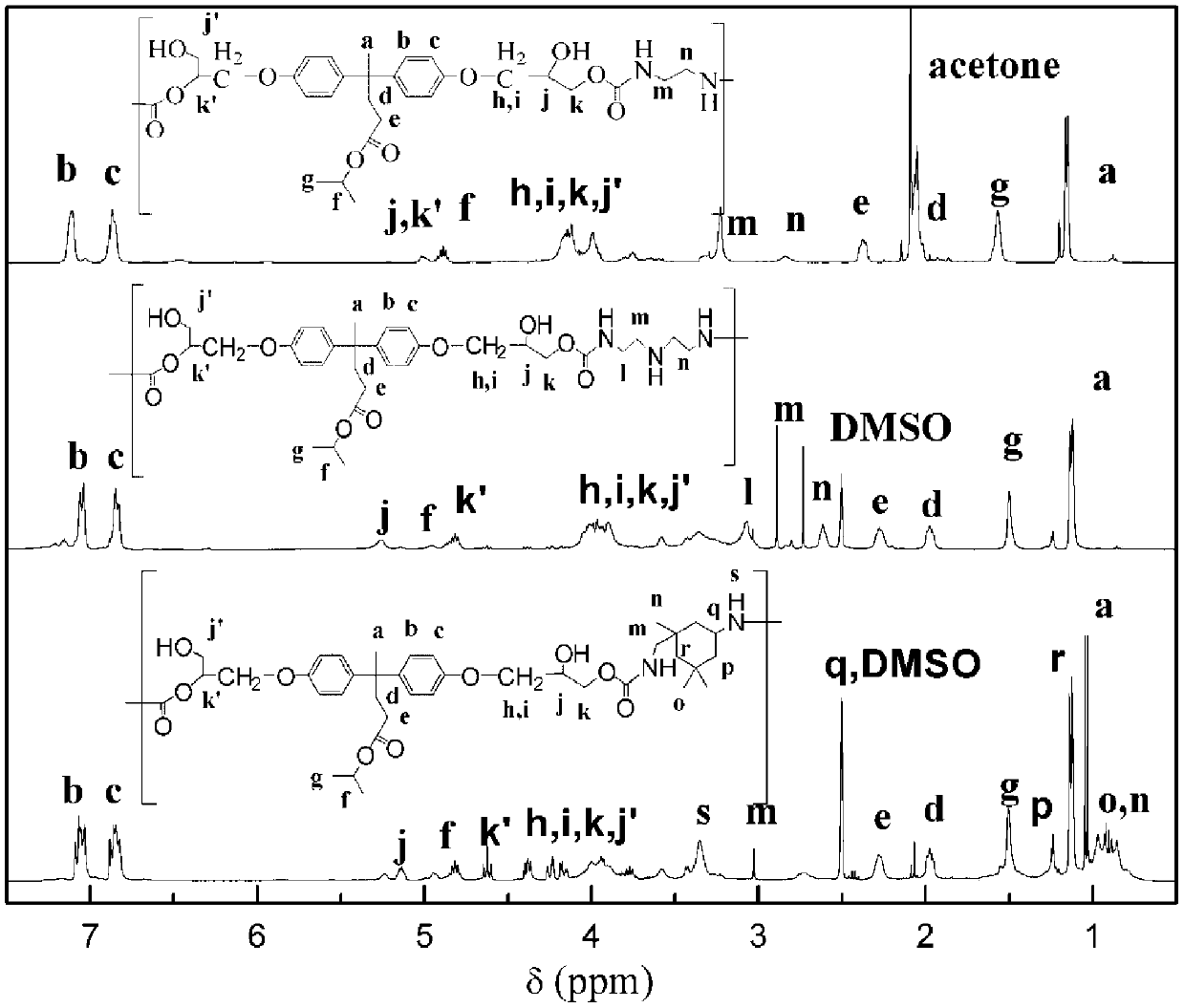
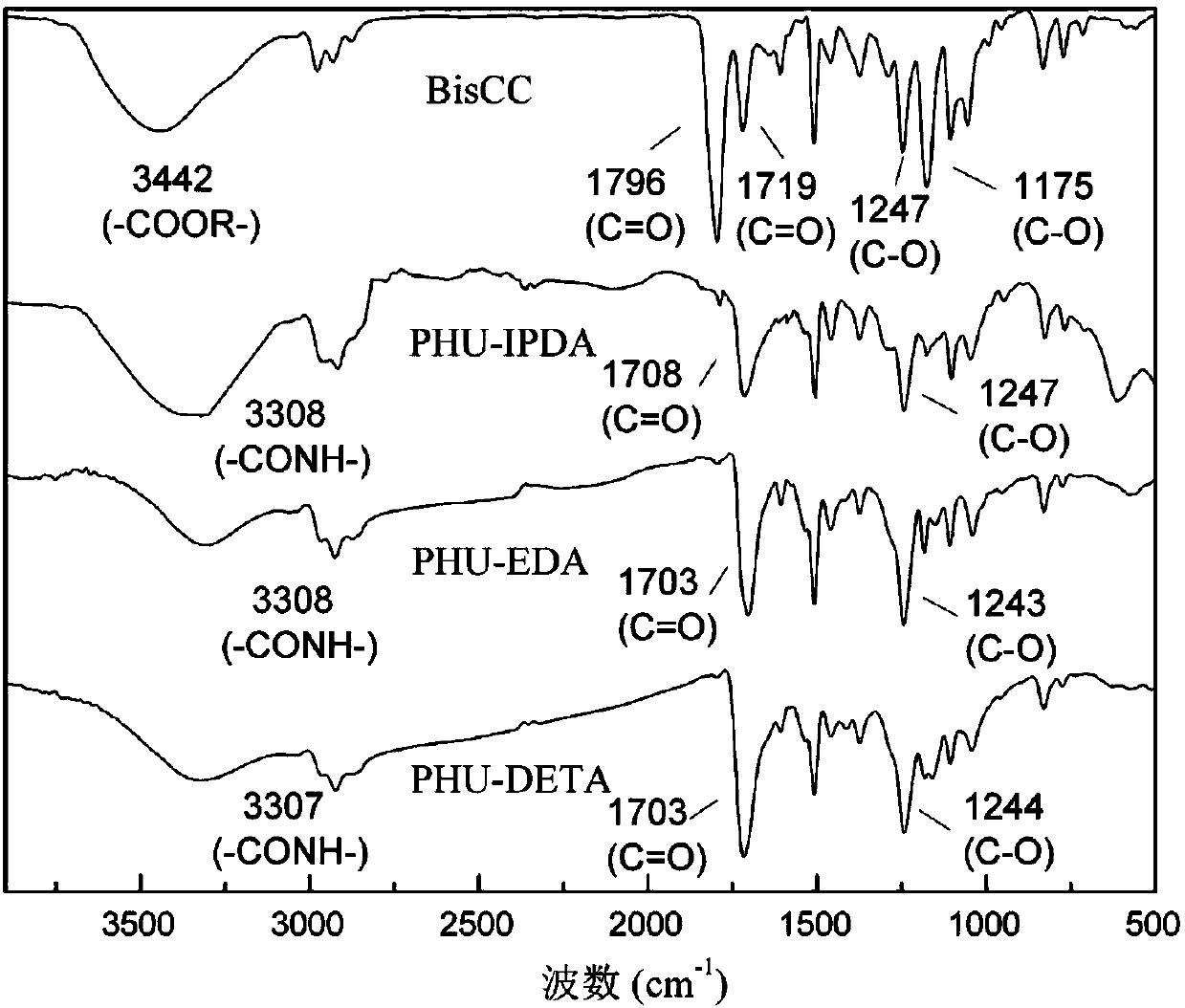
[0091] The method shown in Example 1 was prepared to obtain bisphenolic acid isopropanol bicyclic carbonate (BisCC). Preparation of step 4 bisphenolic acid-based hydroxyl polyurethane (PHU-EDA)
[0092] Add 5.28g of bisphenolic acid-based bicyclic carbonate, 0.60g of ethylenediamine and 10mL of solvent N-pyrrolidone into a 20mL round-bottomed flask, stir and heat up to 80°C. After reacting for 8 hours, the solvent was removed in vacuo to obtain a tan hydroxyl polyurethane solid with a molecular weight of about 4500.
[0093] step 5
[0094] Mix 0.5g of bisphenolic acid-based hydroxyl polyurethane (PHU-EDA) and 0.33g of E51 epoxy resin (NH- to epoxy group equivalent ratio 1:1), dissolve in 1mL of acetone, and ultrasonically disperse for one minute. The solution was coated on the surface of an aluminum sheet (20mm×30mm×1mm). After the acetone is evaporated to form a paint film, the aluminum sheet is put into an oven for stepwise heating and curing (60°C for...
Embodiment 3
[0097] Step 1~3
[0098] The method shown in Example 1 was prepared to obtain bisphenolic acid isopropanol bicyclic carbonate (BisCC). The preparation of step 4 bisphenolic acid-based hydroxyl polyurethane (PHU-IPDA)
[0099] Add 5.28g of bisphenolic acid-based bicyclic carbonate, 1.70g of isophoronediamine and 10mL of solvent N-pyrrolidone into a 20mL round bottom flask, stir and heat up to 80°C. After reacting for 8 hours, the solvent was removed in vacuo to obtain a tan hydroxyl polyurethane solid with a molecular weight of about 4600.
[0100] step 5
[0101] Mix 0.5g of bisphenolic acid-based hydroxyl polyurethane (PHU-IPDA) and 0.28g of E51 epoxy resin (NH- to epoxy group equivalent ratio 1:1), dissolve in 1mL of acetone, and ultrasonically disperse for one minute. The solution was coated on the surface of an aluminum sheet (20mm×30mm×1mm). After the acetone is evaporated to form a paint film, the aluminum sheet is put into an oven for stepwise heating and curing (60...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com