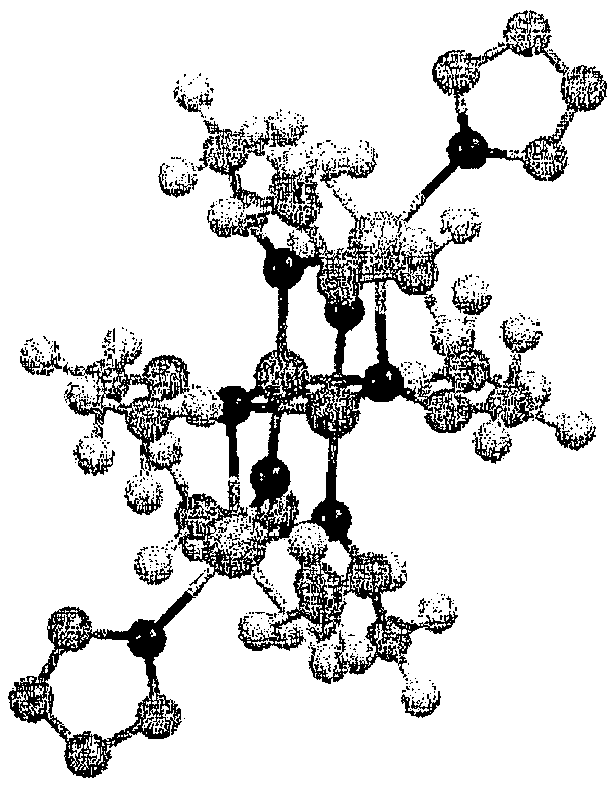
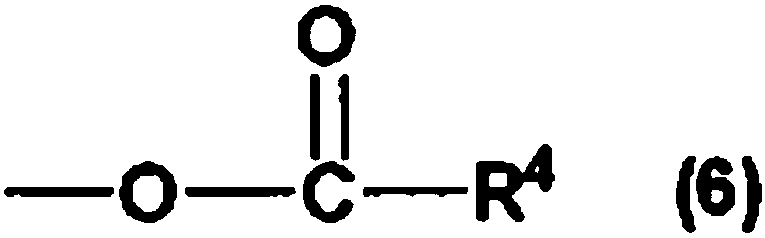
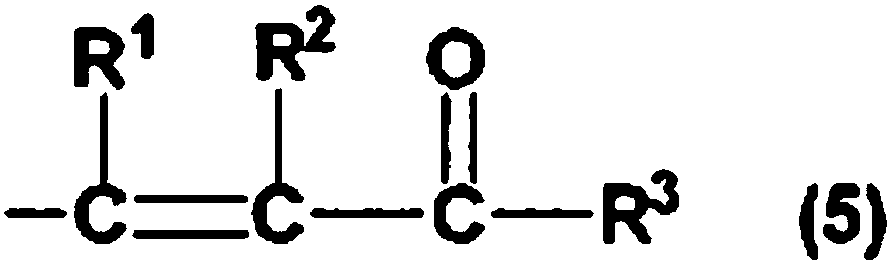Hydrosilylation reaction catalyst
A technology of hydrosilylation and catalyst, applied in catalytic reaction, physical/chemical process catalyst, organic compound/hydride/coordination complex catalyst, etc., can solve the problem of low stability of compound, lack of selectivity, and degree of effect ambiguity etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0382] Hereinafter, synthesis examples, examples, and comparative examples are given to describe the present invention more specifically, but the present invention is not limited to the following examples.
[0383] All solvents used in the preparation of metal compounds were deoxidized and dehydrated by known methods.
[0384] The obtained metal compound was stored at 25°C under a nitrogen atmosphere and used for the reaction.
[0385] The hydrosilylation reaction of olefins and the purification of solvents were all carried out under an inert gas atmosphere, and solvents used in various reactions were all previously purified, dried, and deoxidized by known methods.
[0386] 1 JNMLA400 manufactured by JEOL Ltd. was used for H-NMR measurement, FT / IR-550 manufactured by JASCO Co., Ltd. was used for IR measurement, and VariMax manufactured by Rigaku Co., Ltd. was used for X-ray crystal structure analysis. MoKα line 0.71069 Ongstrom proceeds.
[0387] In addition, in the chemica...
Synthetic example 1
[0389] [Synthesis example 1] Synthesis of iron pivalate
[0390] With reference to the document J.Cluster Sci., 2005, 16, 331., it was synthesized by the following method.
[0391] 0.86 g of reduced iron (15.4 mmol, manufactured by Kanto Chemical Co., Ltd.), and 3.50 g of pivalic acid (34.3 mmol, manufactured by Tokyo Chemical Industry Co., Ltd.) were added to a 50 mL two-necked eggplant-shaped flask with a reflux tube. , stirred at 160°C for 12 hours. At this time, the reaction solution changed from colorless and transparent to green. Furthermore, 2.50 g (24.5 mmol) of pivalic acid was added, and it stirred at 160 degreeC for 19 hours. Thereafter, the reaction solution was filtered, combined with the recovered supernatant, and dried at 80° C. under reduced pressure. The resulting solid was treated with Et 2 O washing gave a green solid (2.66 g, 67% yield).
[0392] FT-IR(KBr)ν:2963,2930,2868,1583,1523,1485,1457,1427,1379,1362,1229,1031,938,900,790,608,576,457cm -1
Synthetic example 2
[0393] [Synthesis example 2] Synthesis of cobalt pivalate
[0394] With reference to the document Russ.Chem.Bull., 1999, 48, 1751., it was synthesized by the following method.
[0395] In a 50 mL two-necked eggplant-shaped flask with a reflux tube, add 1.15 g of cobalt acetate (6.5 mmol, manufactured by Wako Pure Chemical Industries, Ltd.), 1.55 g of pivalic acid (15.2 mmol), and 0.5 g of pivalic anhydride. mL (2.5 mmol, manufactured by Tokyo Chemical Industry Co., Ltd.), stirred at 160° C. for 1 hour. At this time, the reaction solution changed from light purple to purple. Afterwards, it was dried under reduced pressure at 80°C, and the obtained solid was washed with pentane and Et 2 O was washed and dried to obtain a purple solid (1.15 g, yield 68%).
[0396] FT-IR (KBr) ν: 2963, 2929, 2868, 1599, 1524, 1485, 1457, 1420, 1379, 1363, 1229, 1032, 938, 900, 792, 613, 585, 460cm -1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com