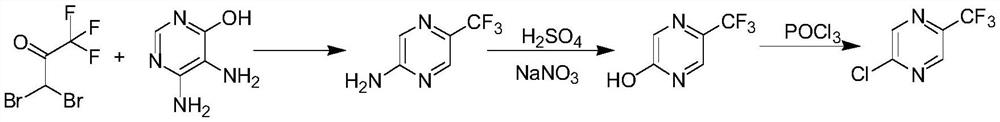
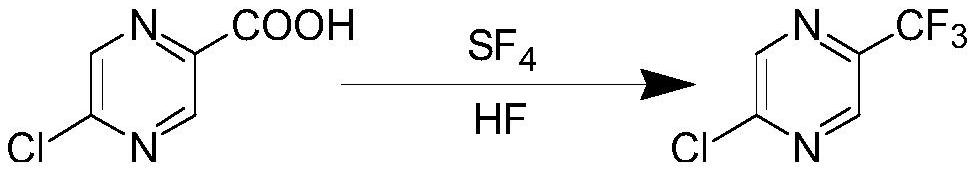
A kind of synthetic method of 2-chloro-5-trifluoromethylpyrazine
A technology of trifluoromethylpyrazine and synthetic method, which is applied in the direction of organic chemistry, can solve the problems of low product yield, low yield, long reaction steps, etc., and achieves easy-to-obtain raw materials, high yield, and simple process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] In a 2L stainless steel autoclave, add 158.5g (1.0mol) of 5-chloropyrazine-2-carboxylic acid, cool down to 0-10°C, add 240g (12mol) of anhydrous hydrogen fluoride, and continue to cool down to -40°C, 238 g (2.2 mol) of sulfur tetrafluoride was introduced. Then slowly raise the temperature to 80-90°C, and keep it warm for 4 hours. After the reaction is completed, the reaction liquid is poured into ice water, neutralized with 10% sodium carbonate aqueous solution to pH = 7, the organic layer is separated, steam distilled, and the obtained product is rectified to obtain 2-chloro-5-tri Fluoromethylpyrazine 146g, content 99%, yield 80%. Its structural characterization is shown in the following data:
[0031] 2-Chloro-5-trifluoromethylpyrazine product structure confirmed: 1H-NMR (400MHz, CDCl3) σ: 8.74ppm (s, 1H); 8.70 (s, 1H).
Embodiment 2
[0033] In a 2L stainless steel autoclave, add 317g (2.0mol) of 5-chloropyrazine-2-carboxylic acid, cool down to 0-10°C, add 480g (24mol) of anhydrous hydrogen fluoride, continue to cool down to -40°C, and Add 476 (4.4mol) g of sulfur tetrafluoride. Then slowly raise the temperature to 75-80°C, and keep it warm for 6 hours. After the reaction is completed, the reaction liquid is poured into ice water, neutralized with 10% sodium carbonate aqueous solution to pH = 7, the organic layer is separated, steam distilled, and the obtained product is rectified to obtain 2-chloro-5-tri Fluoromethylpyrazine 329g, content 99%, yield 90%. Its structural characterization is shown in the following data:
[0034] 2-Chloro-5-trifluoromethylpyrazine product structure confirmed: 1H-NMR (400MHz, CDCl3) σ: 8.74ppm (s, 1H); 8.70 (s, 1H).
Embodiment 3
[0036] In a 2L stainless steel autoclave, add 317g (2.0mol) of 5-chloropyrazine-2-carboxylic acid, cool down to 0-10°C, add 300g (15mol) of anhydrous hydrogen fluoride, continue to cool down to -40°C, and Add 476 (4.4mol) g of sulfur tetrafluoride. Then slowly raise the temperature to 75-80°C, and keep it warm for 6 hours. After the reaction is completed, the reaction liquid is poured into ice water, neutralized with 10% sodium carbonate aqueous solution to pH = 7, the organic layer is separated, steam distilled, and the obtained product is rectified to obtain 2-chloro-5-tri Fluoromethylpyrazine 274g, content 99%, yield 75%. Its structural characterization is shown in the following data:
[0037] 2-Chloro-5-trifluoromethylpyrazine product structure confirmed: 1H-NMR (400MHz, CDCl3) σ: 8.74ppm (s, 1H); 8.70 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com