Adriamycin and gene medicine co-transporting nano medicine carrying system and preparation method
A gene drug and nano-drug loading technology, applied in drug combination, gene therapy, pharmaceutical formula, etc., can solve the problem of inability to completely and effectively reverse tumor MDR, achieve sensitization and toxicity, improve chemotherapy efficacy, and improve stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The present invention also provides a preparation method of the above-mentioned doxorubicin and gene drug co-delivery nano-loading system, comprising the following steps:
[0033]Firstly, the membrane material is prepared into liposome colostrum by thin film hydration method. In the present invention, there is no special limitation on the specific operation steps of the thin film hydration method, and methods well known to those skilled in the art can be used. In the present invention, it is preferred that the liposomal colostrum step is specifically: the chloride salt of 1,2-dioleoyl-3-trimethylammonium-propane, dipalmitoylphosphatidylcholine, TPGS and Cholesterol is dissolved in chloroform according to the above-mentioned prescription ratio, and the chloroform is removed under reduced pressure to form a dry lipid film. Ammonium sulfate solution is added to the dry lipid film, and the liposome colostrum is formed by hydration in a water bath at 40-50°C.
[0034] In th...
Embodiment 1
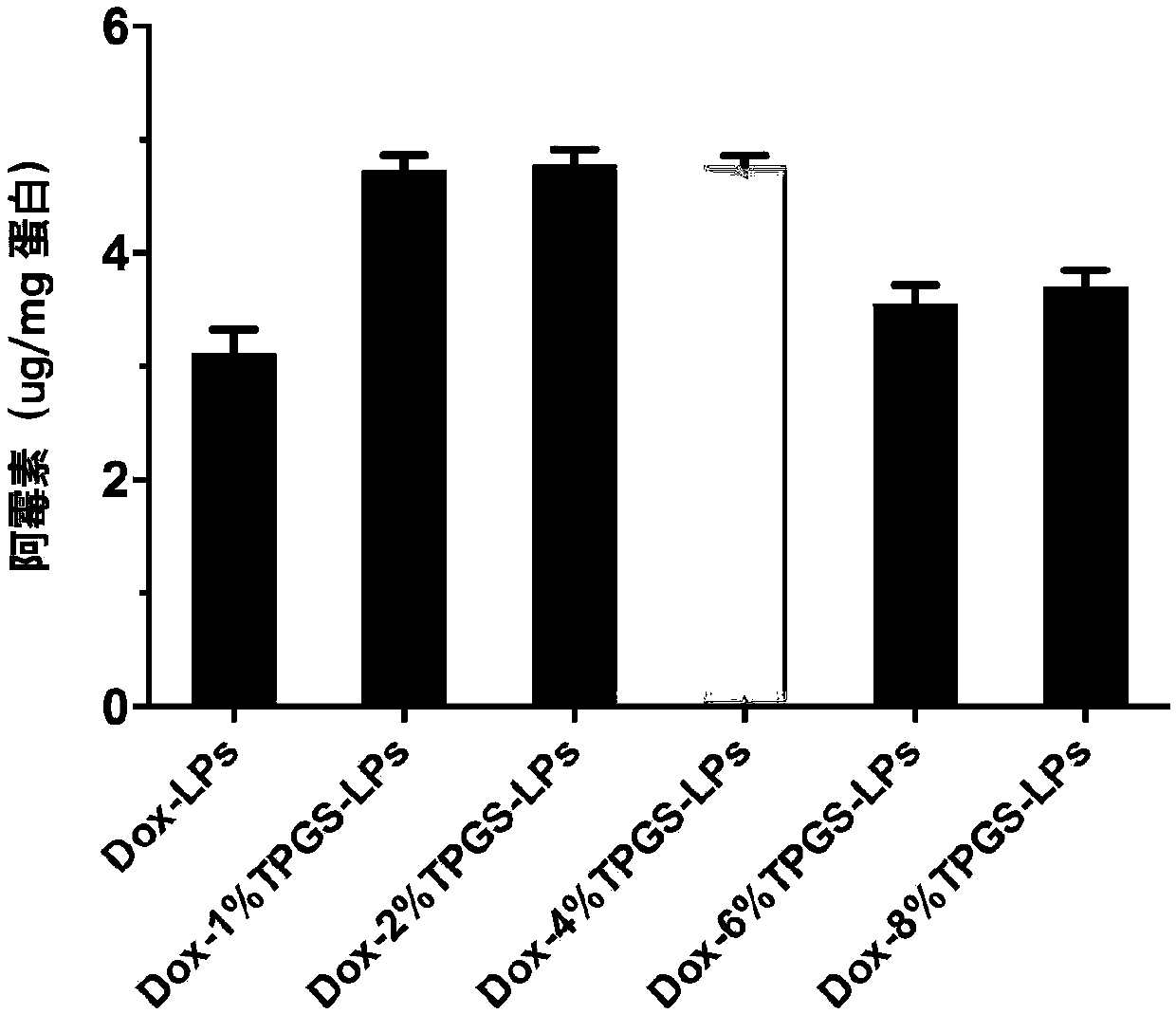
[0051] Preparation of blank cationic liposomes (TPGS-LPs) with 1% TPGS content
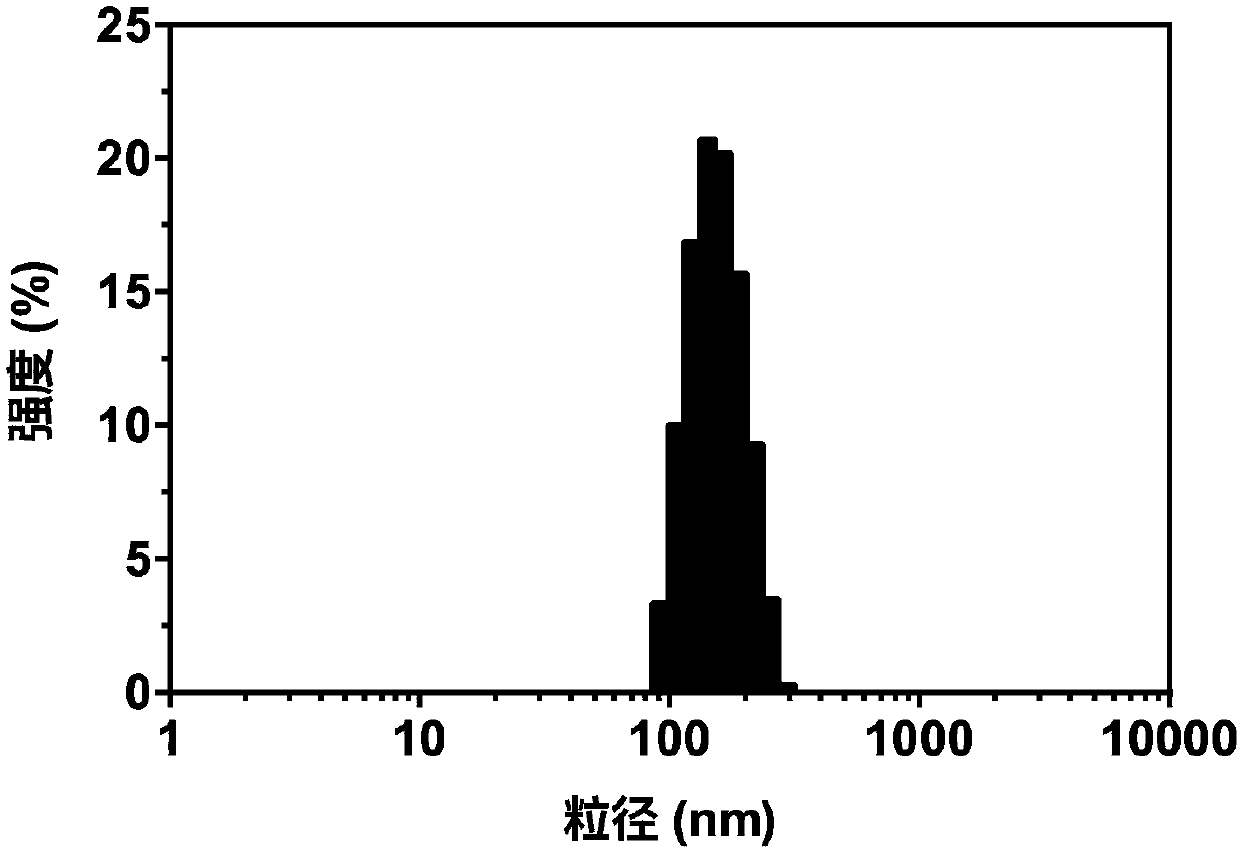
[0052] Dissolve 4.22mg of DOTAP, 4.44mg of DPPC, 0.23mg of TPGS and 1.11mg of cholesterol in 4mL of chloroform and place in a 50mL round bottom flask, and remove the chloroform by rotary evaporation under a reduced pressure of 0.1MPa and a water bath at 40°C to form a dry lipid film; add 1mL of 123mM ammonium sulfate solution was hydrated in a water bath at 40°C for 1 hour to obtain liposome colostrum; liposome colostrum was passed through a 100nm polycarbonate membrane 19 times using a micro-liposome extruder to obtain 1% TPGS- LPs blank liposomes, the TPGS content is 1% based on the total lipid mole percentage.
Embodiment 2
[0054] Preparation of blank cationic liposomes (TPGS-LPs) with 2% TPGS content
[0055] Dissolve 4.15mg DOTAP, 4.36mg DPPC, 0.45mg TPGS and 1.04mg cholesterol in 4mL chloroform and place in a 50mL round-bottom flask, and remove the chloroform by rotary evaporation under 0.1MPa decompression and 40°C water bath to form a lipid dry film; add 1mL of 123mM ammonium sulfate solution was hydrated in a water bath at 40°C for 1 hour to obtain liposome colostrum; liposome colostrum was passed through a 100nm polycarbonate membrane 19 times using a micro-liposome extruder to obtain 2% TPGS- LPs blank liposomes, the TPGS content is 2% based on the total lipid mole percentage.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| electric potential / voltage | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com