Preparation method of triamcinolone
A synthesis method and compound technology, applied in steroids, organic chemistry and other directions, can solve the problems of expensive raw materials, high production cost, many by-products, etc., and achieve the improvement of product yield, production cost saving, and by-product reduction. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
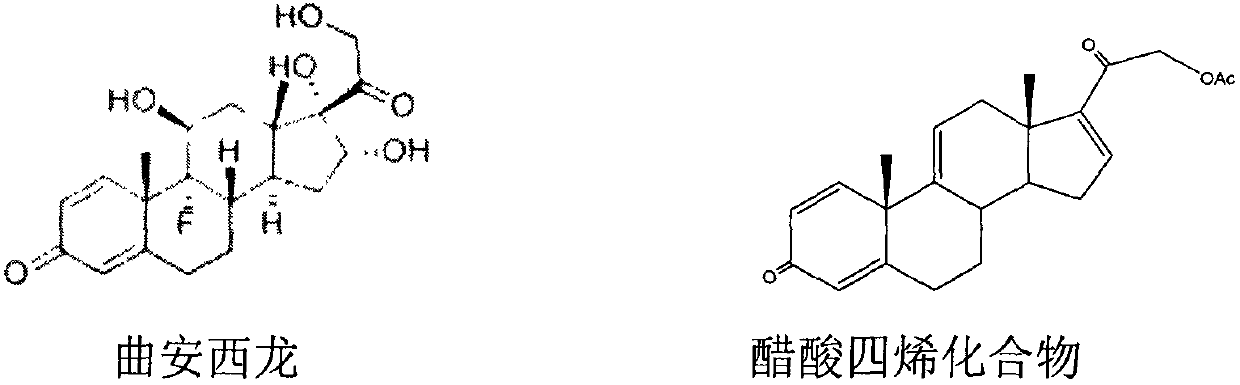
[0032] The invention discloses a preparation method of triamcinolone, and those skilled in the art can learn from the content of this article and appropriately improve the process parameters to realize it. It needs to be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all considered to be included in the present invention, and relevant personnel can obviously make changes without departing from the content, spirit and scope of the present invention. Changes or appropriate changes and combinations are made to the content described herein to realize and apply the technology of the present invention.
[0033] In the present invention, unless otherwise specified, the scientific and technical terms used herein have the meanings commonly understood by those skilled in the art.
Embodiment 1
[0035] Embodiment 1: the preparation of formula II compound
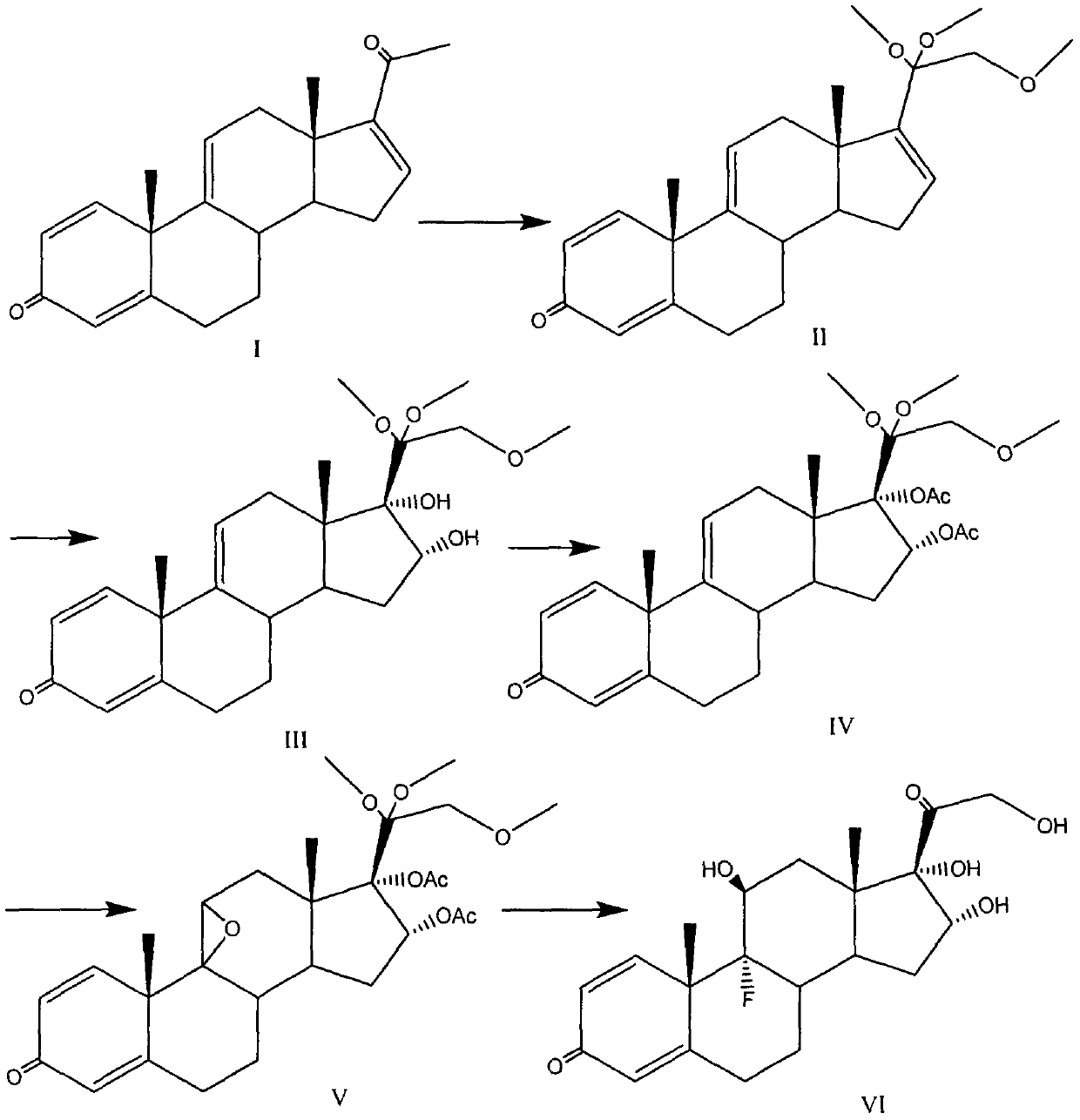
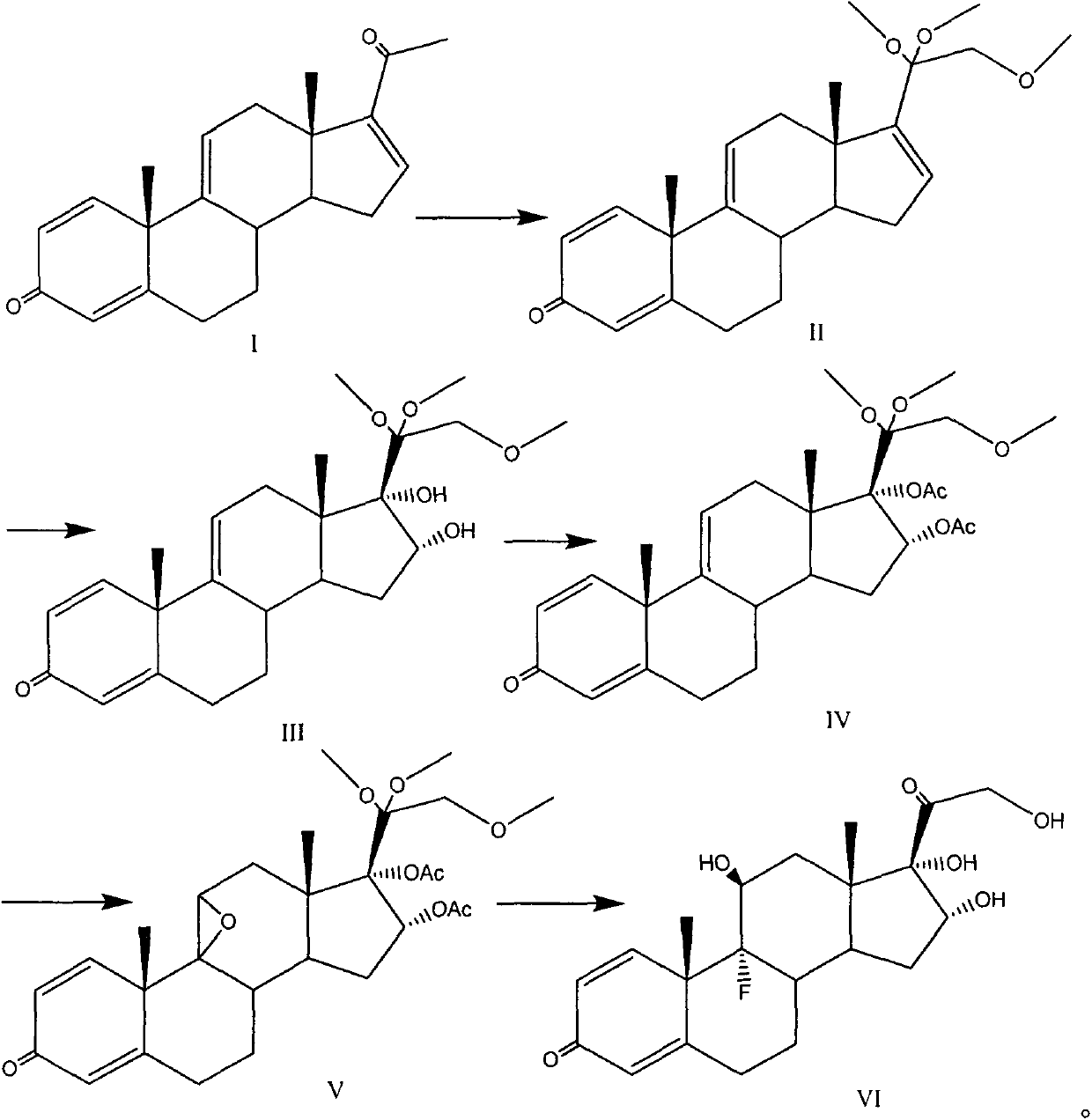
[0036]
[0037] At a temperature of -5 to 5°C, 100mL of methanol is used as a solvent, and the reaction bottle is placed in an ice-water bath to control the low temperature. 5.4 grams (17.5 mmol) of compound I and 4.2 grams (105 mmol) of sodium hydroxide are added to the reaction bottle, and stirred for 10- 30min, under dark conditions, add 8.5g (26.3mmol) iodobenzene diacetate at one time, naturally rise to room temperature and react for 6-8 hours, TLC monitors the reaction process, add 20g (141mmol) methyl iodide dropwise, continue the reaction, wait After the reaction was completed, the solvent was removed under reduced pressure, extracted with 100 mL of ethyl acetate and 20 mL of water, the organic phase was washed with water, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain 6.3 grams (16.3 mmol) of the compound of formula II with a yield of 93 %.
Embodiment 2
[0038] Embodiment 2: the preparation of formula III compound
[0039]
[0040] 150mL of acetone was used as a solvent, 7.1g (18.4mmol) of the compound of formula II was added, the temperature was lowered to -5~5°C, 2.8mL of about 3.4g (73.4mmol) of formic acid was added as a catalyst, and 5.2g (33.1mmol) of potassium permanganate was added Oxidation reaction, TLC monitors the reaction process. After the reaction is completed, excess potassium permanganate is treated with saturated sodium sulfite solution, insoluble solids are removed by filtration, washed with acetone, decolorized by activated carbon, solids are removed by filtration, the solvent is removed under reduced pressure, and recrystallization with pure water yields formula III Compound 6.8 g, yield 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com