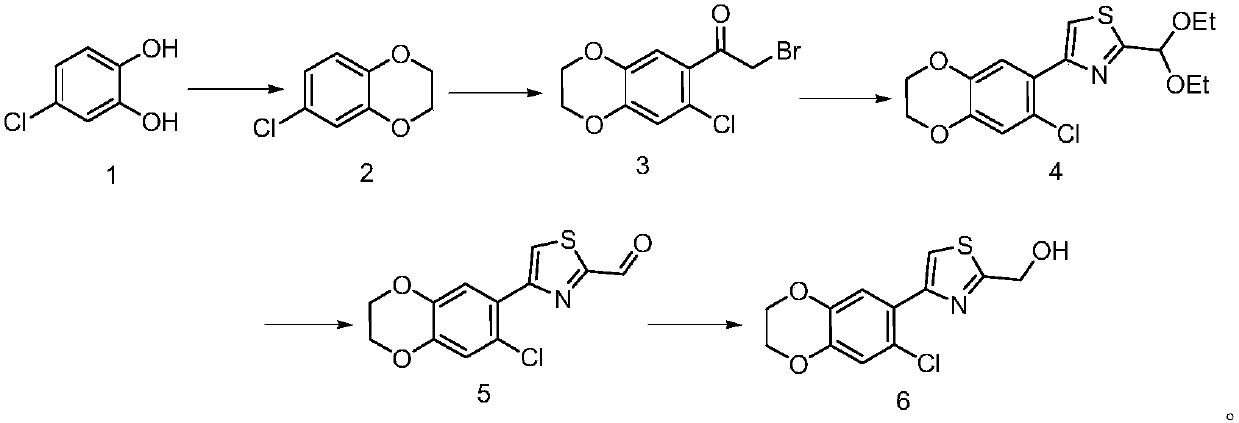
Preparation method of thiazole derivative
A technology for thiazoles and products, which is applied in the field of new preparation of pharmaceutical intermediates, and can solve problems such as difficult synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
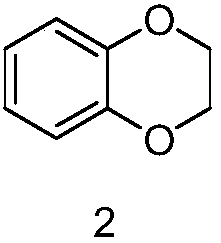
[0023] (1) Synthesis of 6-chloro-2,3-dihydrobenzo[b][1,4]dioxine
[0024] Add 30g of 4-chlorocatechol to 400ml of N,N-dimethylformamide, add 21g of anhydrous potassium carbonate, then add 35g of 1,2-dibromoethane, heat under reflux and stir for 15 hours, then cool to room temperature , concentrated to remove most of N,N-dimethylformamide, added water and ethyl acetate, extracted, separated, dried, concentrated, and the residue was separated on a column to obtain 38g of 6-chloro-2,3-dihydrobenzo [b][1,4]dioxine.
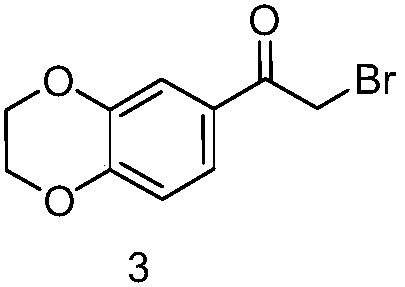
[0025] (2) Synthesis of 2-bromo-1-(7-chloro-2,3-dihydrobenzo[b][1,4]dioxin-6-yl)ethane
[0026] Add 38g of 6-chloro-2,3-dihydrobenzo[b][1,4]dioxine into 500ml of dichloromethane, add 60g of bromoacetyl bromide, heat and reflux for 4 hours, and cool to At room temperature, add water again, extract and separate, collect the organic phase, separate, dry, concentrate, and separate the residue on a silica gel column to obtain 42g of 2-bromo-1-(7-chloro-2,3-dihydrobenzo[b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com