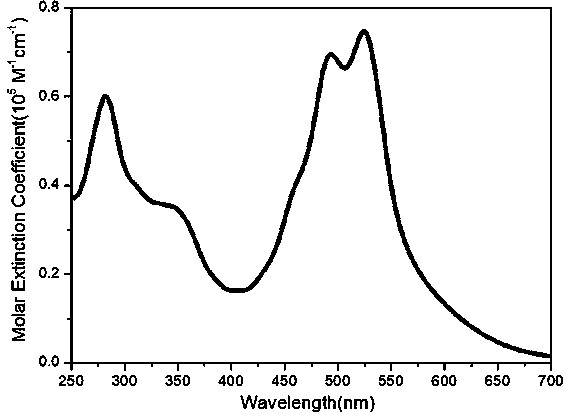
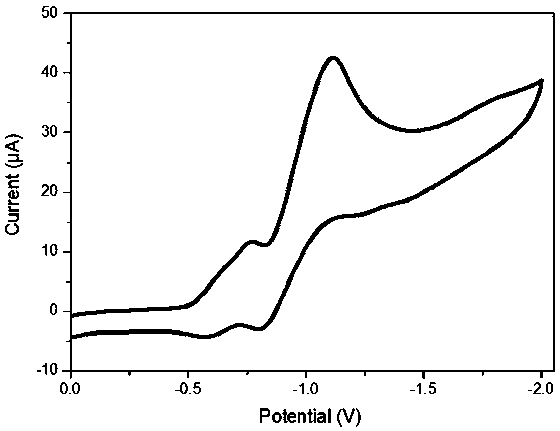
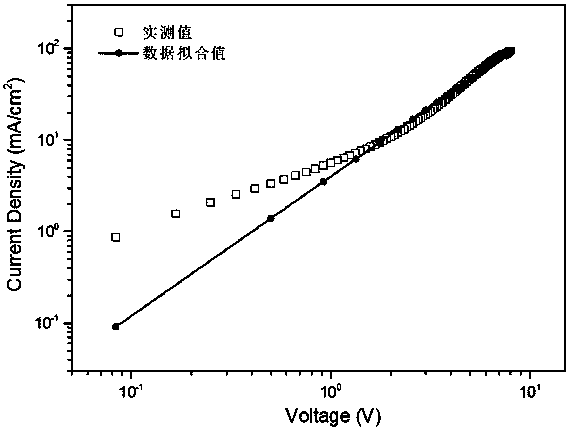
Triphenyldioxinimide diploid compound and preparation method thereof
A technology of triphenyl dioxazine imide and monobromotriphenyl dioxazine imide, which is applied in the field of triphenyl dioxazine imide diploid compound and preparation thereof, and can solve the problem of limiting the application and development of dyes , poor solubility, unfavorable solution processing and other problems, to achieve the effect of good redox characteristics and excellent electron transport performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
[0031] Monobromotriphenyldioxazinimide (2.00mmol), bistributyltinthiophene (1.00mmol) and Pd 2 (dba) 3 (0.1 mmol) was added into the reactor, and toluene was used as a solvent, and the reaction was heated at 80° C. for 12 h. The end point of the reaction was determined by thin-plate chromatography, and the reaction was stopped. Liquid dichloromethane and water, washed with water, dried, desolvated, and purified by silica gel column chromatography to obtain a purple solid, yield: 72%, HRMS: 2049.3177 (m / z).
Embodiment 2
[0033]
[0034] Monobromotriphenyldioxazinimide (5.00mmol), bistributyltindithiophene (1.00mmol) and Pd 2 (dba) 3 (0.2 mmol) was added into the reactor, chlorobenzene was used as a solvent, and the reaction was heated at 100° C. for 16 h. The end point of the reaction was determined by thin-plate chromatography, and the reaction was stopped. Liquid dichloromethane and water, washed with water, dried, desolvated, and purified by silica gel column chromatography to obtain a purple solid, yield: 78%, HRMS: 1966.9487 (m / z).
Embodiment 3
[0036]
[0037] Monobromotriphenyldioxazinimide (3.00mmol), bisborate triphenylamine (1.00mmol), Pd(PPh 3 ) 4 (0.05mmol), 2M K 2 CO 3 An aqueous solution (4.00 mmol) was added into the reactor, ethylene glycol monomethyl ether was used as a solvent, and the reaction was heated at 120° C. for 8 hours. The end point of the reaction was determined by thin-plate chromatography, and the reaction was stopped. Liquid dichloromethane and water, washed with water, dried, desolvated, and purified by silica gel column chromatography to obtain a purple solid, yield: 82%, HRMS: 2162.1345 (m / z).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Optical bandgap | aaaaa | aaaaa |
| Electron mobility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com