Transglutaminase mutant expressed in active form
A glutamine and mutant technology, applied in the field of enzyme engineering, can solve problems such as inability to meet requirements, and achieve the effects of easy separation and purification, easy cultivation, and simple fermentation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The construction of embodiment 1 genetically engineered bacteria po1h / hpro-mTG
[0049] The plasmid pINA1297 / N355Q reserved in the laboratory was used as a template, and P1 and P2 were used as primers to carry out PCR, and the 1297 expression vector containing the hpro zymogen region was amplified by PCR. The PCR amplification system is: template 1 μL, upstream and downstream primers 1 μL, primeSTAR 25 μL, double distilled water 22 μL. The PCR conditions are: 98°C for 3min, 98°C for 10s, 60°C for 5s, 72°C for 5min30s, 72°C for 20min, 30 cycles. The plasmid pET 20b / mpro-mTG reserved in the laboratory was used as a template, and P3 and P4 were used as primers to carry out PCR, and the gene fragment containing mTG was amplified by PCR. The PCR amplification system is the same as above, and the PCR conditions are: 98°C for 3min, 98°C for 10s, 60°C for 5s, 72°C for 1min 20s, 72°C for 10min, 30 cycles. The two PCR products were digested by Dpn I and recovered from the gel. ...
Embodiment 2
[0052] Determination of embodiment 2 mutation sites
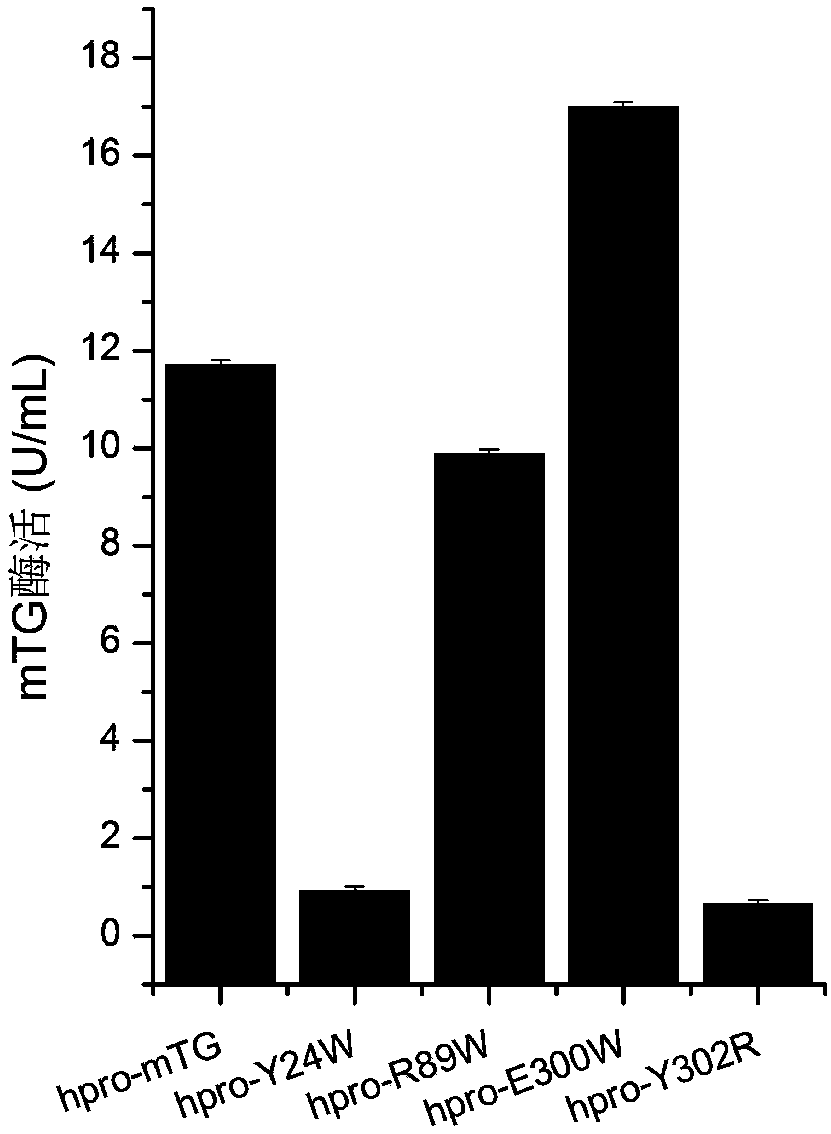
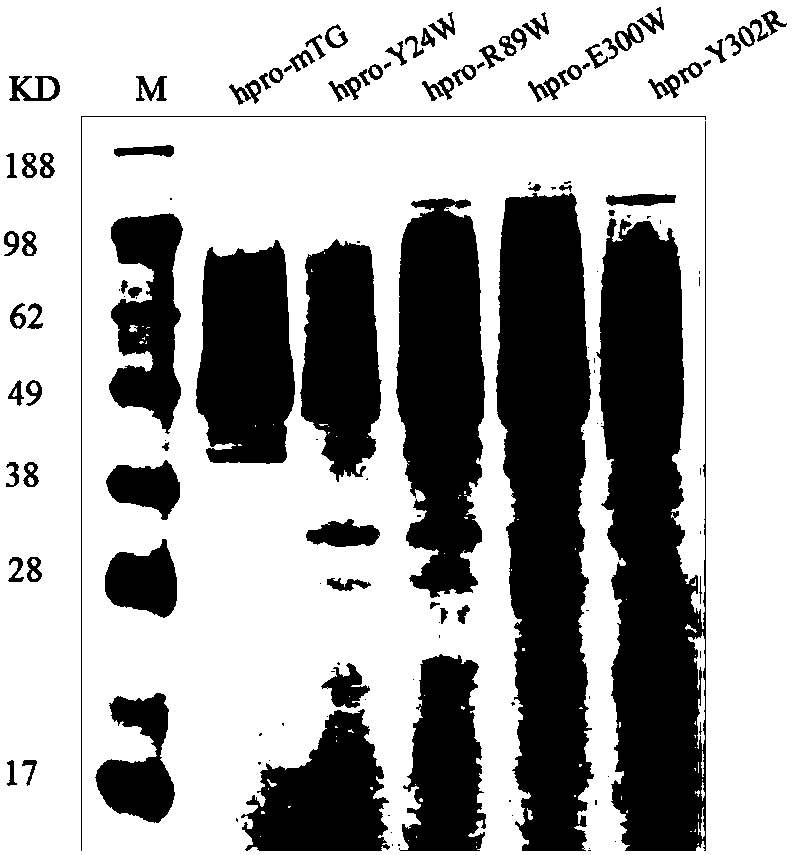
[0053] Use Discovery Studio2017 software to carry out virtual amino acid mutations, determine the key amino acids in the active site, and perform targeted mutations on the sites that affect the affinity between the enzyme and the substrate according to the prediction results, namely Y24W, R89W, E300W, and Y302R.
Embodiment 3
[0054] Example 3 Construction of site-directed mutagenesis expression strain
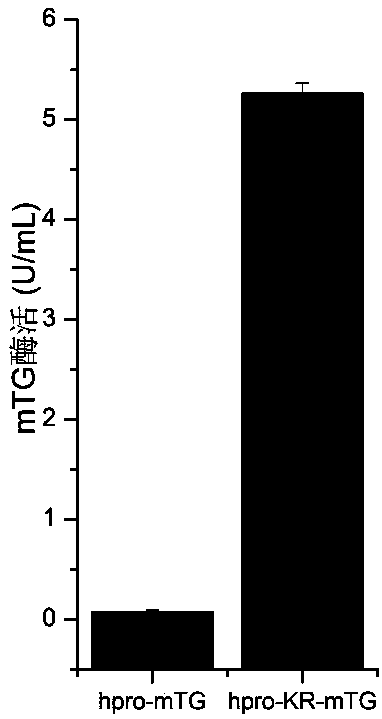
[0055] (1) The transglutaminase gene derived from Streptomyces Maoyuan and the zymogen region hpro gene derived from Streptomyces hygroscopicus were fused and ligated using the One Step Cloning Kit to construct plasmid pINA1297 / hpro-mTG.
[0056] (2) Use the plasmid pET 20b / mpro-mTG reserved in the laboratory as a template, and use P5 and P4, P6 and P4, P3 and P7, P3 and P8 as primers to carry out PCR, and amplify by PCR to obtain a protein containing Y24W, R89W, E300W, The mutant gene fragment of Y302R. The PCR amplification system is: template 1 μL, upstream and downstream primers 1 μL, primeSTAR 25 μL, double distilled water 22 μL. The PCR conditions were: 98°C for 3min, 98°C for 10s, 60°C for 5s, 72°C for 1min, 72°C for 10min, 30 cycles. After the two PCR products were digested by Dpn I, they were gel-recovered separately. The plasmid pINA297 / hpro-MTG was used as a template, and the recovered...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com