Automatic headspace sampling gas chromatography method for determining chloroethane and chloroform residual in chlortetracycline hydrochloride
A technology of gas chromatography and aureomycin hydrochloride, which is applied in the field of automatic headspace sampling gas chromatography, can solve the problems of manpower consumption, material resources, long detection time, low sensitivity, etc., to save carrier gas, reduce detection cost, The effect of improving test efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Precision Test
[0064] 1. Instrument precision test
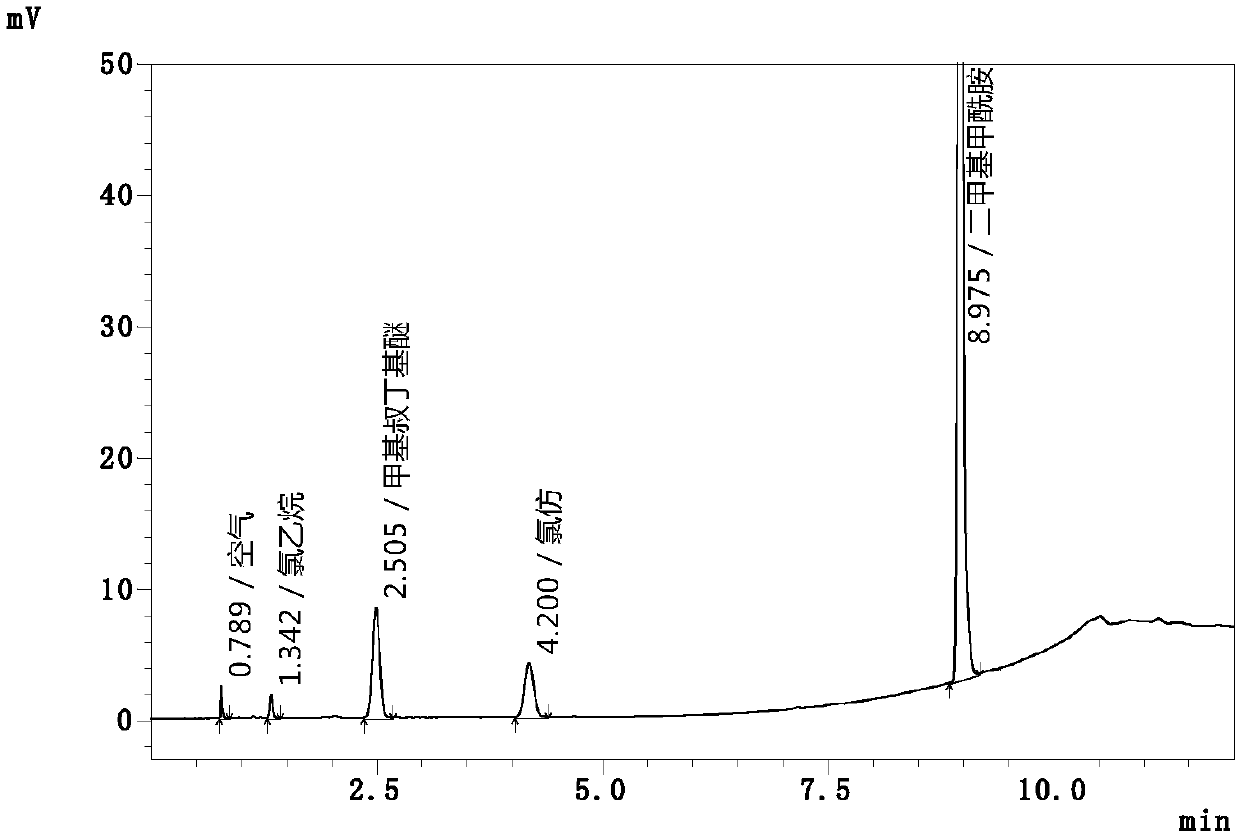
[0065] The precision of the instrument is the embodiment of the repeatability of the instrument. Accurately measure 10mL of the above-mentioned mixed control solution, place it in a 20mL headspace bottle, seal the bottle, and prepare 6 copies in this way, and set them under the above-mentioned gas chromatography conditions and headspace sampling parameters respectively. Under measure, calculate the average value and relative standard deviation (RSD) of ethyl chloride and chloroform peak area, the results are shown in Table 1, wherein the gas chromatogram of parallel experiment group 1 is as follows figure 1 shown.
[0066] Table 1 Instrument precision test data statistics
[0067] serial number
Area of ethyl chloride
1
8108
29644
2
7832
30715
3
8301
29736
4
7794
30402
5
8284
29543
6
7956
29956
ave...
Embodiment 2
[0078] accuracy test
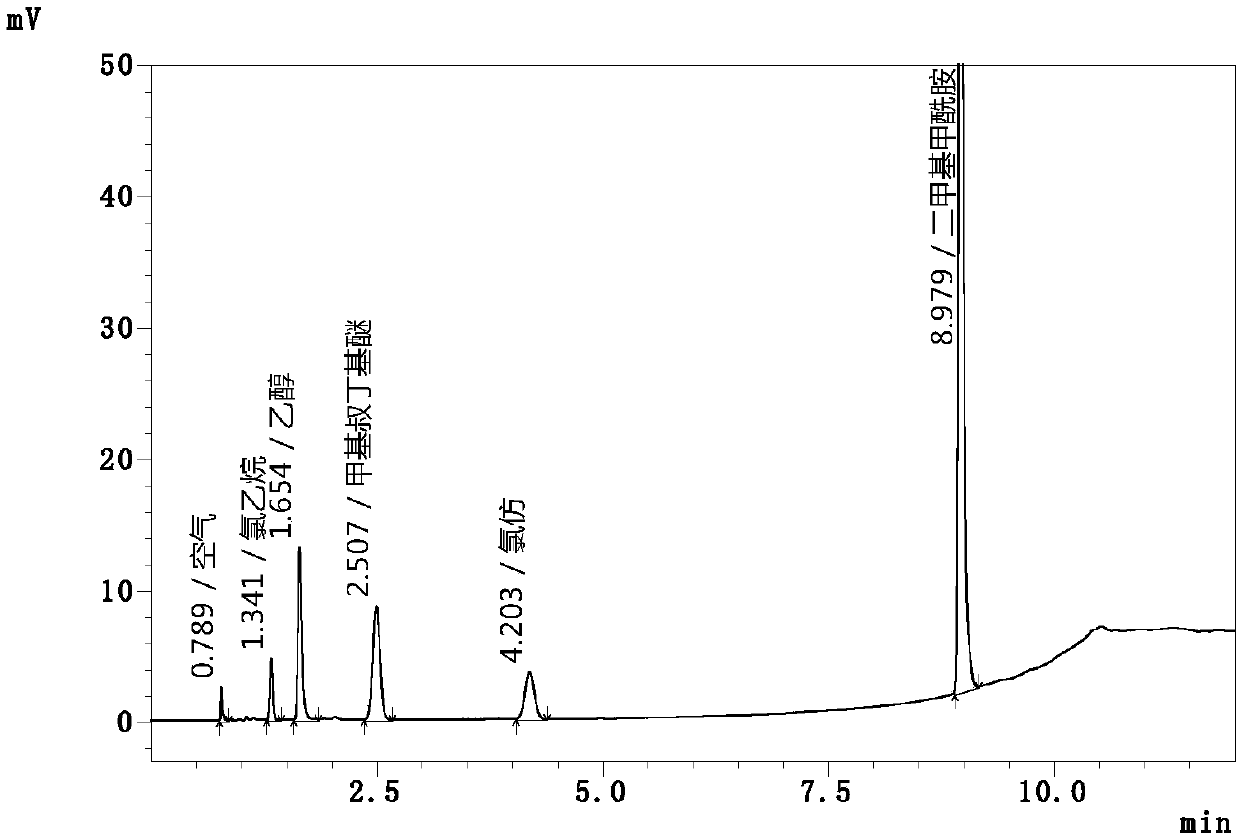
[0079] Accuracy is the closeness between the measured value and the true value, and the accuracy of the method is usually expressed by the recovery rate. Add ethyl chloride and chloroform to aureomycin hydrochloride samples by standard addition method to prepare solutions of three concentration levels, detect the concentration of the standard addition solution, and calculate the recovery rate of each concentration.
[0080] 1. Preparation of recovery standard solution
[0081] Accurately measure 5mL, 10mL and 15mL of the above-mentioned mixed control solutions respectively, place them in a 100mL volumetric flask, add water to dilute to the mark, and obtain 50% recovery solution, 100% recovery solution and 150% recovery solution respectively, and then take 10mL50 %, 100% and 150% of the recovery solution, each 3 parts, were added to 20ml headspace vials, and then 0.05g chlortetracycline hydrochloride sample was added to 9 headspace vials, after the chlor...
Embodiment 3
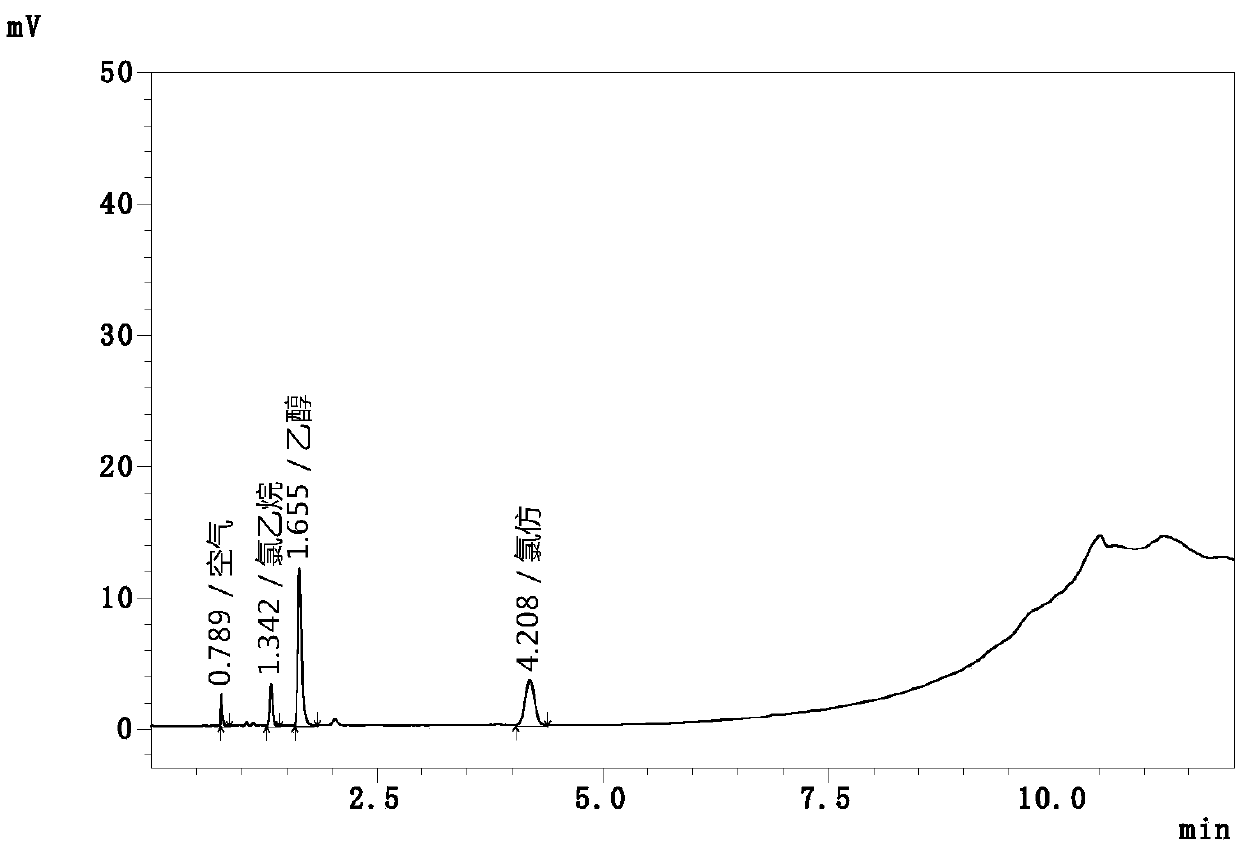
[0086] Linearity and range determination
[0087] Precisely measure 1mL, 2mL, 5mL, 10mL, 15mL, 20mL, 40mL, 50mL of the mixed standard stock solution respectively and place them in a 100mL volumetric flask, dilute to the mark with water to obtain a series of mixed standard solutions of 8 concentration levels, ethyl chloride and Chloroform concentrations were approximately 0.01 μg / mL, 0.02 μg / mL, 0.05 μg / mL, 0.10 μg / mL, 0.15 μg / mL, 0.20 μg / mL, 0.40 μg / mL, 0.50 μg / mL.
[0088] Get each 10mL of above-mentioned series mixed contrast solution, place respectively in the headspace bottle of 20mL, seal the bottle, measure respectively according to above-mentioned this gas chromatographic condition and headspace sampling parameter setting, record the peak area of ethyl chloride and chloroform, The results are shown in Table 4, carry out linear regression analysis with the amount of ethyl chloride and chloroform detected in this series of mixed contrast solutions and the corresponding ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com