Single-domain antibody capable of recognizing HLA-A2/RMFPNAPYL
A technology of HLA-A2 and single-domain antibody, applied in the field of single-domain antibody that recognizes HLA-A2/RMFPNAPYL, can solve the problem of lack of literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
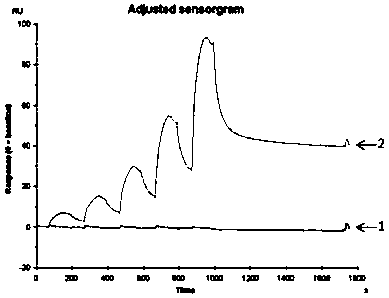
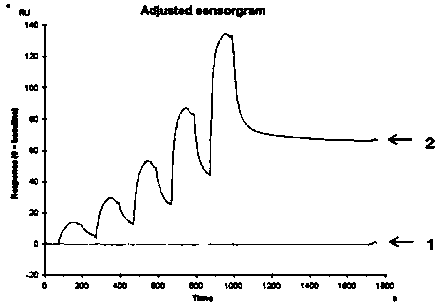
Image
Examples
Embodiment 1
[0051] Example 1 Screening single domain antibodies of HLA-A2 / RMFPNAPYL complex
[0052] 1.1 Preparation of single-domain antibody phage library
[0053] 1.1.1 Preparation of helper phage (BM13)
[0054] The M13KE phage replicon was double digested with AlwnI and AfeI, and the synthetic gene fragment was also double digested with AlwnI and AfeI, and then ligated together with T4 ligase. After ligation, TG1 was transfected to obtain helper phage BM13. Thus, in the original replicator
[0055] The tctggtggtggttctggtggcggctctgagggtggtggctctgagggtggcggttctgagggtggcggctctgagggaggcggttccggtggtggctct sequence was replaced by a synthetic gene sequence, that is, a trypsin cleavage sequence was added to the phage GIII coding region. Once used as a helper phage, a trypsin digestion step was added to reduce the number of phages that did not contain the fusion target gene protein.
[0056] The synthetic gene sequence is as follows:
[0057] CCA GCC GGC CTT TCT GAG GGG TCG ACT ATA GAA G...
Embodiment 2
[0102] Example 2. Expression of single domain antibody
[0103] Prokaryotic single domain antibody expression
[0104] The four single-domain antibody genes were cloned into pET22b with Nco I and Not I restriction enzymes before and after, respectively, to express the monovalent antibody. The constructed vector was transformed into E.coli / DE3, single clone was picked the next day, shaken at 37°C 220rpm and cultured to an OD600 of about 0.5, after adding IPTG (working concentration of 1mM), induced expression at 18°C 220rpm for 20h. Detection of protein expression. The sonicated supernatant was purified with ProteinA and then run SDS-PAG, see Figure 4 . Among them, Marker's strips are 14, 25, 30, 40, 50, 70, 100, 120, 160KD from small to large. Line1 is M5-H1, line2 is M5-G3, and line3 is M5-F4. Single domain antibodies are about 14KD in size.
[0105] Expression of FC fusion in pET22b In order to form a diabody, the single domain antibody gene was linked to human FC t...
Embodiment 3
[0112] Example 3. Specific recognition of HLA-A2 / RMFPNAPYL complex by single domain antibody
[0113] Specific Recognition of Antigen Complexes by Single Domain Antibodies
[0114] The single-domain antibody expressed on pET22b was collected, resuspended in PBS, and then sonicated. Ultrasonication conditions: 600W, ultrasonic for 2 seconds, interval of 6 seconds, 10 minutes in total, 16°C. After sonication, centrifuge at 12,000 rpm at 4°C for 10 minutes, take the supernatant for ELISA identification of the specificity of different antigens (Protein A-HRP is the secondary antibody) and read the plate. For data analysis, see Figure 9 . Among them, the ordinate is the light absorption value at 650 nm, and the abscissas 1, 2, 3, and 4 are the four antigens HLA-A2 / ITDQVPFSV, HLA-A2 / NLVPMVATV, HLA-A2 / RMFPNAPYL, HLA-A2 / SLLMWITQC. Three samples were sonicated supernatants of M5-H1, M5-G3, M5-F4 expressed in pET22b alone.
[0115] The results showed that the single-domain antibody...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Affinity constant | aaaaa | aaaaa |
| Affinity constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com