A multifunctional nickel complex based on organic ligands and its method for synthesizing carbon paste electrodes and the application of carbon paste electrodes
A technology of nickel complexes and organic ligands, applied in the direction of nickel organic compounds, material electrochemical variables, etc., can solve the problems of antibiotic loss and antibiotic failure, and achieve enhanced affinity, increased hydrophilicity, and good space to expand space Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1 synthesizes [Ni(L)(H 2 O) 4 ]·(2,5-TPD), where L is N,N'-bis(4-methylenepyridine)benzene-1,4-dicarboxamide, the structural formula is: 2,5-TPD is 2,5-thiophenedicarboxylate
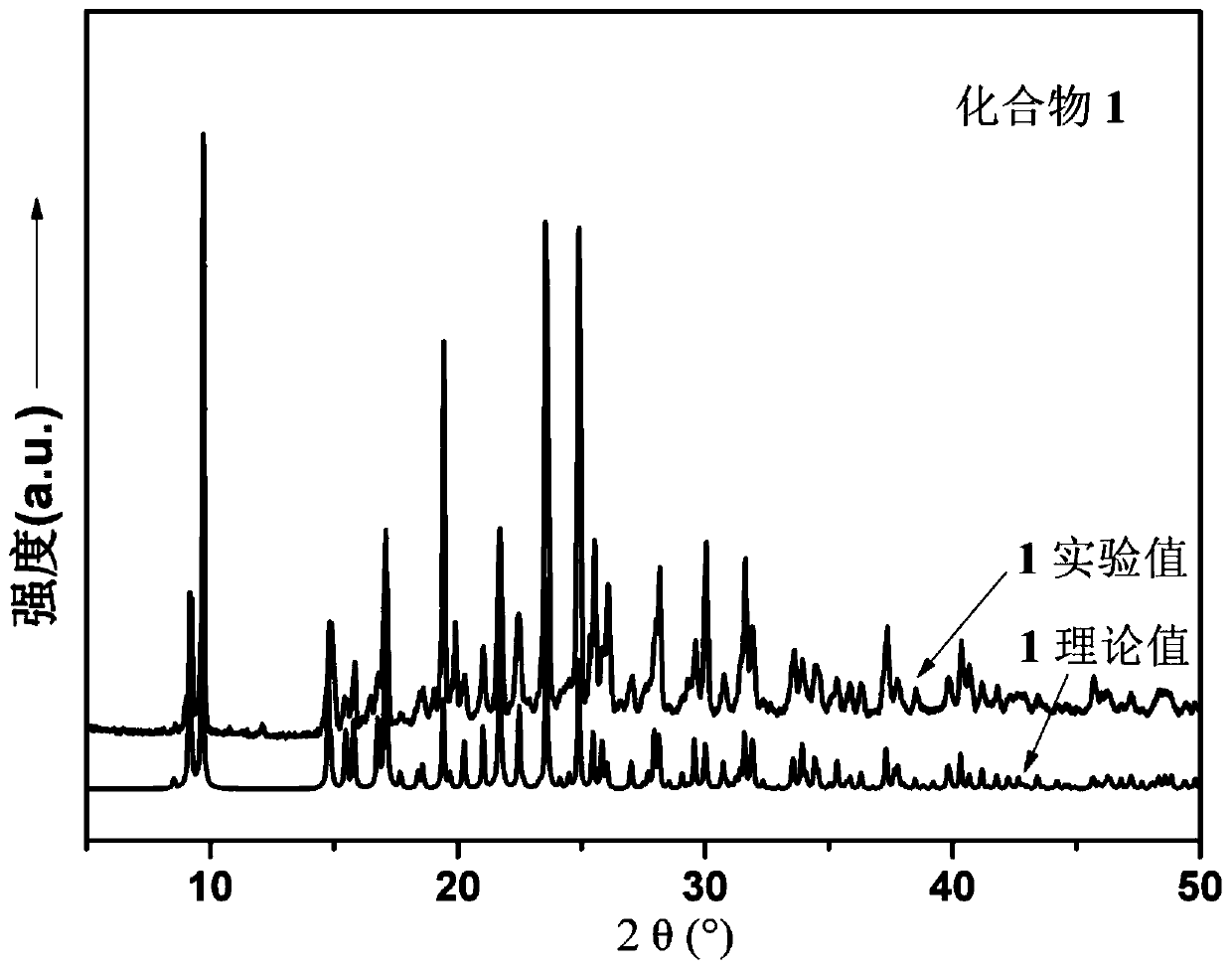
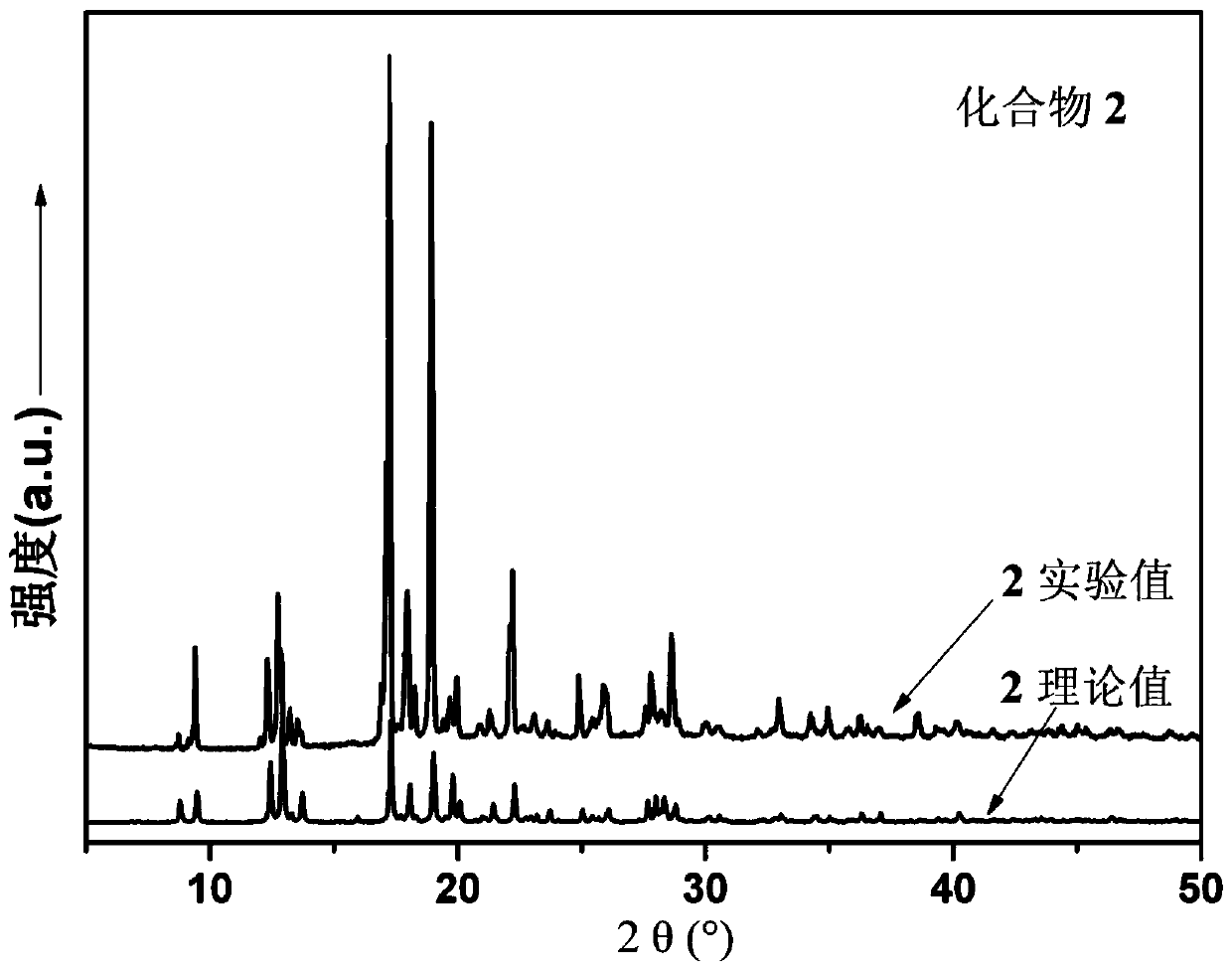
[0044] 0.1mmol NiCl 2 ·6H 2 O, 0.10 mmol N,N'-bis(4-methylenepyridine)benzene-1,4-dicarboxamide, 0.1 mmol 2,5-thiophenedicarboxylic acid, and 5 mL H 2 O was sequentially added to a 25mL beaker, stirred at room temperature for 20min to obtain a suspension mixture, adjusted the pH of the suspension mixture to 4.2 with 1mol / L NaOH solution, then transferred to a 25mL autoclave, heated at 5°C / h The temperature was raised to 110°C, kept under hydrothermal conditions for 48 hours, and the temperature was lowered to room temperature at a cooling rate of 5°C / h to obtain pink blocky crystals, which were washed alternately with deionized water and ethanol for 3 times, and dried naturally at room temperature. Get [Ni(L)(H 2 O) 4 ]·(2,5-TPD), the yield is 45%, and its XRD diffraction patte...
Embodiment 2
[0046] Embodiment 2 synthesizes [Ni(L)(H 2 O) 4 ]·(2,5-TPD), where L is N,N'-bis(4-methylenepyridine)benzene-1,4-dicarboxamide, and 2,5-TPD is 2,5-thiophene di Formate
[0047] 0.2mmol NiCl 2 ·6H 2 O, 0.10 mmol N,N'-bis(4-methylenepyridine)benzene-1,4-dicarboxamide, 0.15 mmol 2,5-thiophenedicarboxylic acid, and 10 mL H 2 O was sequentially added to a 25mL beaker, and stirred at room temperature for 40min to obtain a suspension mixture. After adjusting the pH of the suspension mixture to 5.5 with 1mol / L NaOH solution, it was transferred to a 25mL autoclave and heated at 5°C / h The temperature was raised to 120°C, kept under hydrothermal conditions for 96 hours, and the temperature was lowered to room temperature at a cooling rate of 5°C / h to obtain pink blocky crystals, which were washed alternately with deionized water and ethanol for 4 times, and dried naturally at room temperature. Get [Ni(L)(H 2 O) 4 ]·(2,5-TPD), the yield is 75%, and its XRD diffraction pattern is as...
Embodiment 3
[0049] Embodiment 3 synthesizes [Ni(L)(H 2 O) 4 ]·(2,5-TPD), where L is N,N'-bis(4-methylenepyridine)benzene-1,4-dicarboxamide, and 2,5-TPD is 2,5-thiophene di Formate
[0050] 0.3mmol NiCl 2 ·6H 2 O, 0.10 mmol N,N'-bis(4-methylenepyridine)benzene-1,4-dicarboxamide, 0.2 mmol 2,5-thiophenedicarboxylic acid, and 15 mL H 2 O was sequentially added to a 25mL beaker, and stirred at room temperature for 60min to obtain a suspension mixture. After adjusting the pH of the suspension mixture to 6.8 with 1mol / L NaOH solution, it was transferred to a 25mL autoclave and heated at 5°C / h The temperature was raised to 130°C, kept under hydrothermal conditions for 120 hours, and the temperature was lowered to room temperature at a cooling rate of 5°C / h to obtain pink blocky crystals, which were washed alternately with deionized water and ethanol for 5 times, and dried naturally at room temperature. Get [Ni(L)(H 2 O) 4 ]·(2,5-TPD), the yield is 55%, and its XRD diffraction pattern is as...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com