A kind of estrogen hapten, complete antigen and preparation method thereof
A complete antigen and hapten technology, which is applied in the field of immunochemistry and detection, can solve the problems of low product purity, long reaction time, and uncontrollable reaction, and achieve high product purity, no need for heat treatment, and the effect of saving reaction substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
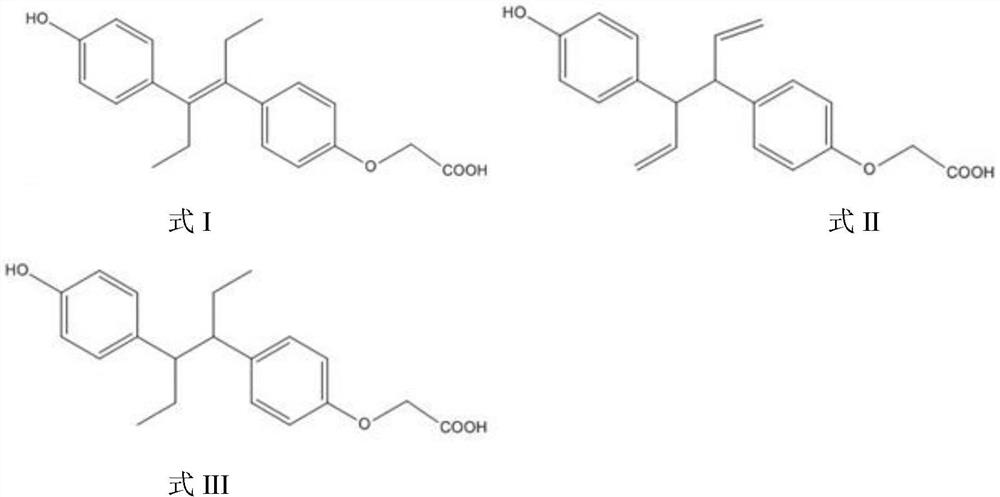
[0018] The estrol hapten is an estrol derivative, and the hapten introduces a carboxyl group on the phenolic hydroxyl side of the estrol to form an estrol-monocarboxymethyl ether compound.
[0019] Specifically, the structural formulas of the estrol haptens prepared in the examples of the present invention are shown in Formula I, Formula II, and Formula III:
[0020]
[0021] As mentioned in the background technology, there are many deficiencies in the existing synthesis of estrol haptens. The present invention redesigns and optimizes the hapten synthesis process, and finally obtains an efficient synthesis method of high-purity estrol haptens.
[0022] In a specific embodiment, the present invention discloses a method for preparing an estrol hapten, the method comprising: carrying out estrol and monochloroacetate in an organic solvent at normal temperature and in the presence of a catalyst. The reaction is terminated with ice water, the unreacted estrols are extracted with ...
Embodiment 1
[0036] Embodiment 1: Preparation method of diethylstilbestrol hapten
[0037] Weigh 295 mg of diethylstilbestrol and place in 6 mL of dry dimethyl sulfoxide, add 1.540 g of potassium hydroxide into the solution, and stir for 5 min. Add 64 mg of sodium monochloroacetate. At room temperature of 10°C, after magnetic stirring for 35 min, 50 mL of ice water was added to terminate the reaction. Add 24 mL of ethyl acetate and extract three times to remove unreacted diethylstilbestrol. The aqueous phase was acidified with 100 mL of 1.5 mol / L hydrochloric acid while stirring, and a white precipitate appeared. Filter through a funnel, wash the precipitate with distilled water until neutral, and dry it in an oven to obtain the diethylstilbestrol hapten with a yield of about 28% and a purity of about 98%.
Embodiment 2
[0038] Embodiment 2: Dienestrol hapten preparation method
[0039] Weigh 293 mg of dienestrol and place in 4 mL of dry dioxane, add 0.924 g of potassium hydroxide into the solution, and stir for 5 min. 128 mg of sodium monochloroacetate was added. At room temperature of 10°C, after magnetic stirring for 35 min, 50 mL of ice water was added to terminate the reaction. Add ethyl acetate 24mL and extract twice to remove unreacted dienestrol. Acidify the aqueous phase with 100mL 2.5mol / L nitric acid while stirring, and a white precipitate appears. Filter through a funnel, wash the precipitate with distilled water until neutral, and dry it in an oven to obtain the dienestrol hapten with a yield of about 29% and a purity of about 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com