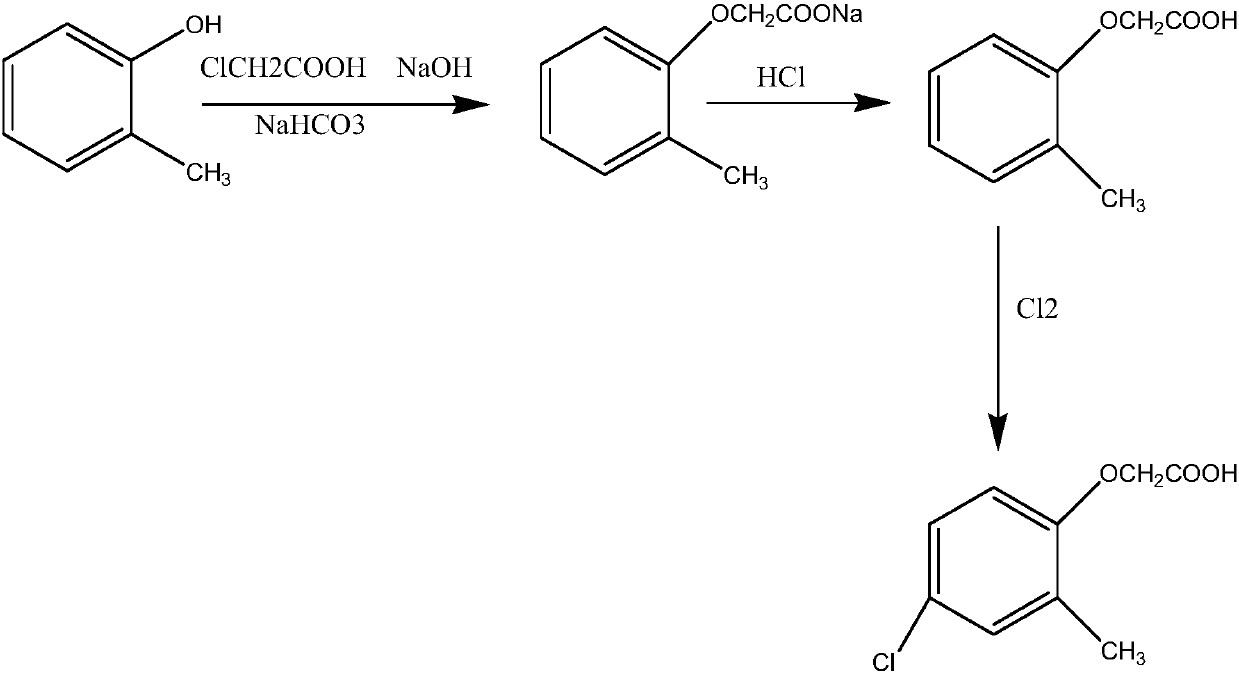
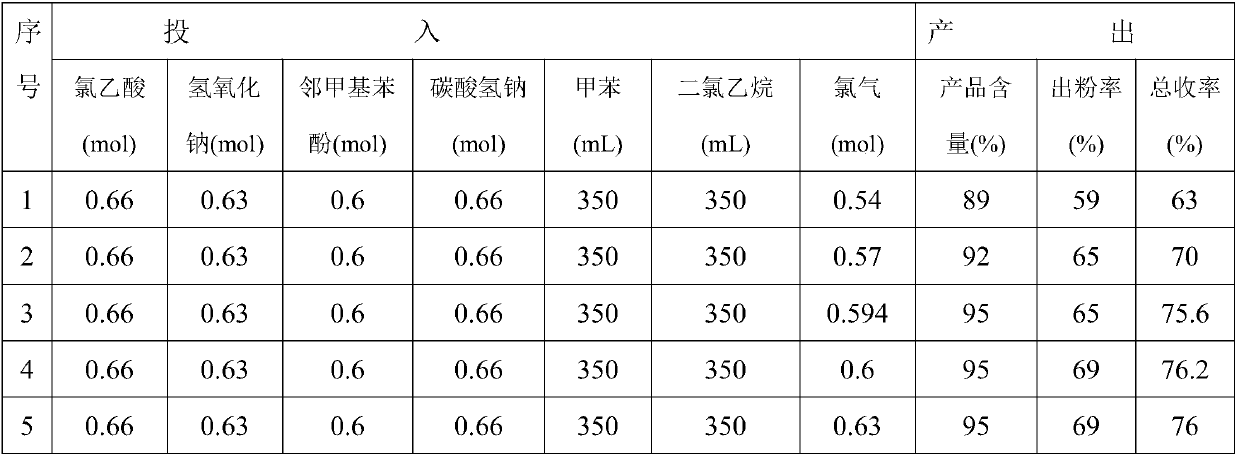
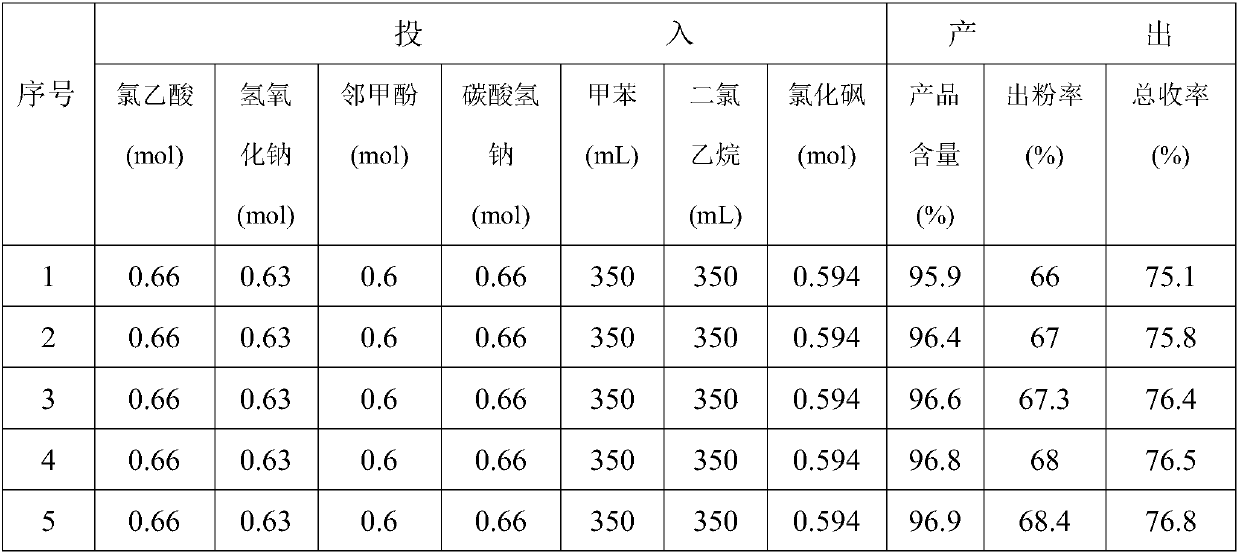
Synthetic method of 2-methyl-4-chlorophenoxyacetic acid
A technology of o-methylphenoxyacetic acid and chlorophenoxyacetic acid, which is applied in chemical instruments and methods, carboxylate preparation, carboxylate preparation, etc., can solve the problem of reducing product content and yield, refractory phenol-containing wastewater, Incomplete neutralization and other problems, to achieve the effect of reducing energy consumption, less three wastes, and less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] A synthetic method of 2-methyl-4-chlorophenoxyacetic acid includes the following steps:
[0020] (1) Preparation of sodium chloroacetate solution: Add quantitative amounts of chloroacetic acid and sodium bicarbonate to a special reactor, mix and stir for 2 hours, and use water at 25°C for cyclic heating. After sampling, add water to completely dissolve it as the end of the reaction. ;
[0021] (2) Preparation of o-methylphenoxyacetic acid: add o-methylphenol into a special reactor, put it in the reactor together with sodium chloroacetate under stirring and heat to reflux, add dropwise 32% sodium hydroxide solution, and Make sure the pH value of the solution is 8-9. After the dripping, continue to dephenolize the water until the temperature rises to 120℃, then start to cool down and add water, and then add hydrochloric acid to adjust the pH of the solution. After the acid adjustment is finished, filter with suction and dry the filter cake to get it. O-methylphenoxyacetic aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com