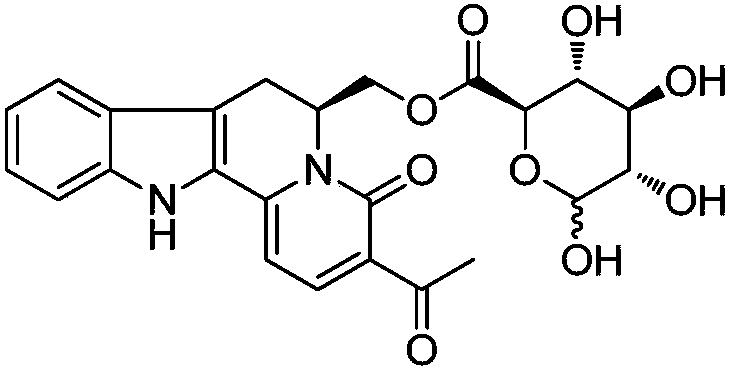
Indole-quinolizine-6-methylol-glucuronate, preparation, activity and application thereof
A technology of furan glucuronate and hydroxymethyl, which is applied in the field of indoquinazine-6-hydroxymethyl-glucuronate, its preparation, activity and application, and can solve the problem of poor prognosis and deterioration of tumor patients The prognosis of cancer patients and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 prepares L-tryptophan methyl ester (1)
[0021] Add 3.8mL (50.0mmol) of thionyl chloride dropwise to 50mL of anhydrous methanol in an ice bath, and the dropwise addition is completed in 30 minutes, then add 9.20g (42.0mmol) of L-tryptophan in batches, and stir at room temperature for 24 hours. TLC monitored disappearance of starting material. The reaction mixture was concentrated to dryness under reduced pressure, and the residue was crystallized from methanol-ether to obtain 10.56 g (92%) of the title compound as a colorless solid.
Embodiment 2
[0022] Example 2 Preparation of (3S)-1-(2,2-dimethoxyethyl)-2,3,4,9-tetrahydro-β-carboline carboxylic acid methyl ester (2)
[0023] Dissolve 6.00g (23.5mmol) of L-tryptophan methyl ester hydrochloride in 30mL of methanol, add 6mL of trifluoroacetic acid, stir for 30 minutes, then add 4.8mL (29.3mmol) of 1,1,3,3-tetramethoxy propane. The temperature was raised to 80° C. for 10 hours under stirring, and the spots of raw materials disappeared as monitored by TLC. The reaction solution was cooled to room temperature, and saturated NaHCO was added dropwise 3 The aqueous solution was adjusted to pH 7. Concentrated under reduced pressure, the residue was extracted with 100mL dichloromethane, anhydrous Na 2 SO 4 dry. After filtration, the filtrate was concentrated to dryness under reduced pressure, and the residue was purified by silica gel column chromatography (petroleum ether: acetone = 2:1) to obtain 2.47 g (33%) of the title compound as a yellow oil. ESI-MS(m / e):319[M+H] ...
Embodiment 3
[0024] Example 3 Preparation of (3S)-1-(2,2-dimethoxyethyl)-3-hydroxymethyl-1,2,3,4-tetrahydro-β-carboline (3)
[0025] Add 0.45g (11.8mmol) LiAlH in batches to 25mL of anhydrous tetrahydrofuran under stirring 4 , and stirred for 0.5 h. It was slowly dropped to 2.47g (7.8mmol) of (3S)-1-(2,2-dimethoxyethyl)-2,3,4,9-tetrahydro-β-carbolinecarboxylic acid methyl ester without In tetrahydrofuran water, the temperature was raised to 40° C. for 3 hours, and the starting point disappeared as monitored by TLC. The reaction solution was cooled to room temperature, and 0.5 mL of 15% NaOH aqueous solution was added to quench the reaction. The aluminum salt was removed by filtration, the filtrate was concentrated to dryness under reduced pressure, and the residue was triturated with ether to obtain 1.10 g (49%) of the title compound as a colorless solid. ESI-MS(m / e):291[M+H] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com