Anti-idiotypic antibody and applications thereof
A technology of antibody and monoclonal antibody, applied in the direction of application, antibody, specific peptide, etc., can solve the problem of undiscovered anti-idiotypic antibody
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115] Example 1: Preparation of anti-idiotypic antibody against monoclonal antibody 11B10
[0116] 1. Preparation of the Fab fragment of monoclonal antibody 11B10
[0117] According to the method described previously (see Chinese patent application 201610382361.6), mAb 11B10 was prepared and purified. The purified monoclonal antibody 11B10 was hydrolyzed with protease, and then the resulting Fab fragment was purified and recovered by ion exchange chromatography, and the protein concentration of the recovered Fab fragment was quantified. In the ion exchange chromatography method, the used affinity chromatography column is the Hitrip column (Pharmacia) of 1 ml, and it is coupled with anti-Fab antibody (anti-Fab-AP, 1:15000 dilution, sigma); The buffer was PBS (pH 7.4), and the elution buffer was 0.2M glycine-HCl (pH 2.4). The eluted product was immediately neutralized with Tris-HCl (1.0 M, pH 8.0) and stored for future use.
[0118] 2. Experimental mice
[0119] Six-week-ol...
Embodiment 2
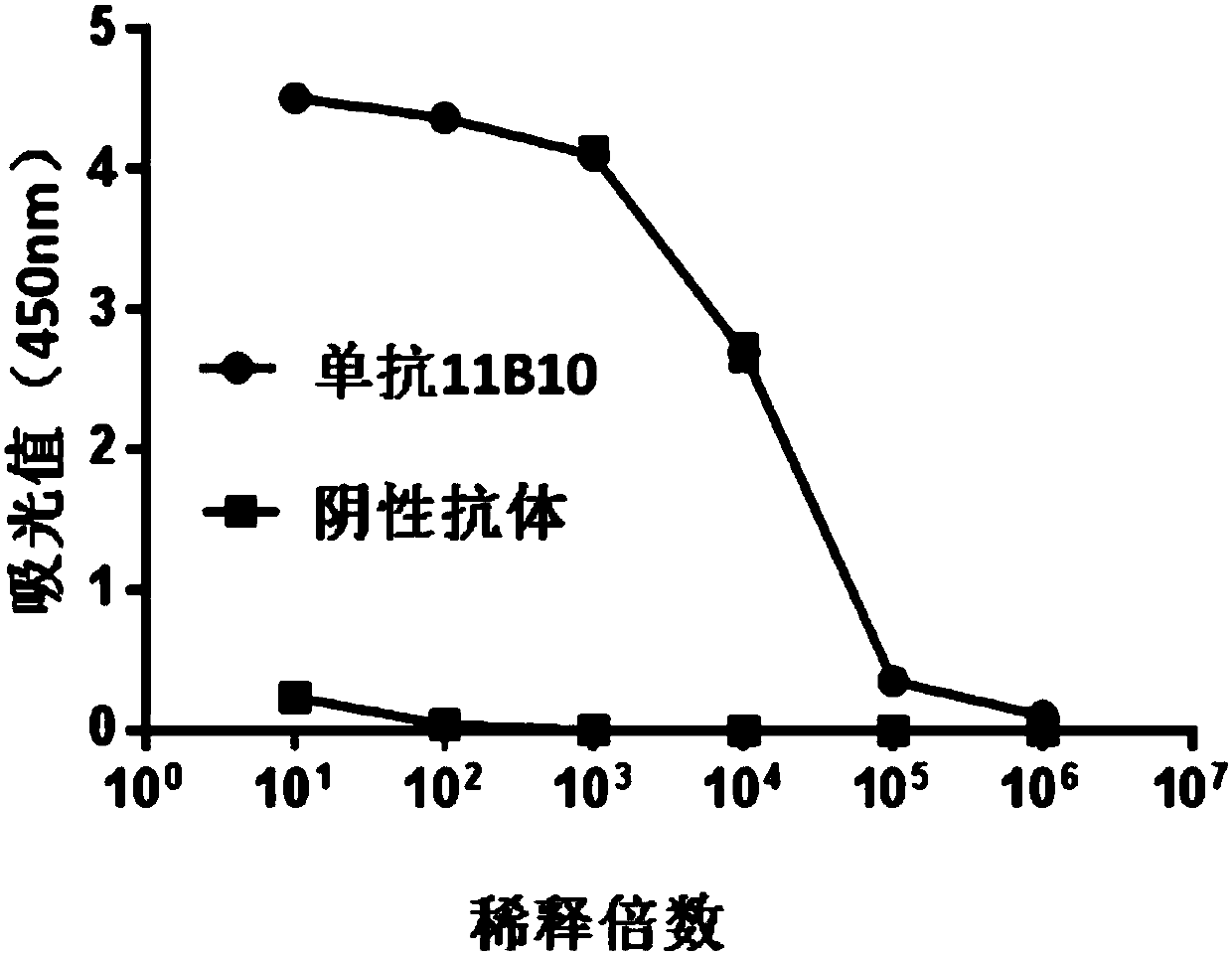
[0139] Example 2: Binding reactivity of anti-idiotypic antibody 1C12 to monoclonal antibody 11B10
[0140] 1. Materials and methods
[0141] Monoclonal antibody 1C12 (200 μl, 100 ng / well) was pre-coated on a 96-well polystyrene microtiter plate. Subsequently, the 96-well plate was blocked with blocking solution. Mab 11B10 was labeled with horseradish peroxidase HRP. The labeled mAb 11B10 was diluted to 0.1 mg / ml as the initial concentration, and then ten-fold serial dilutions (5 times) were performed. The diluted monoclonal antibody 11B10 was added to the above microtiter plate at a volume of 100 μl per well, and incubated at 37° C. for 30 minutes. After incubation, the ELISA plate was washed 5 times with PBST, and then the color developing reagent was added to develop the color for 20 min. Subsequently, read the A450 absorbance value of each well of the microplate plate on a microplate reader. In addition, parallel experiments were performed using an irrelevant antibody ...
Embodiment 3
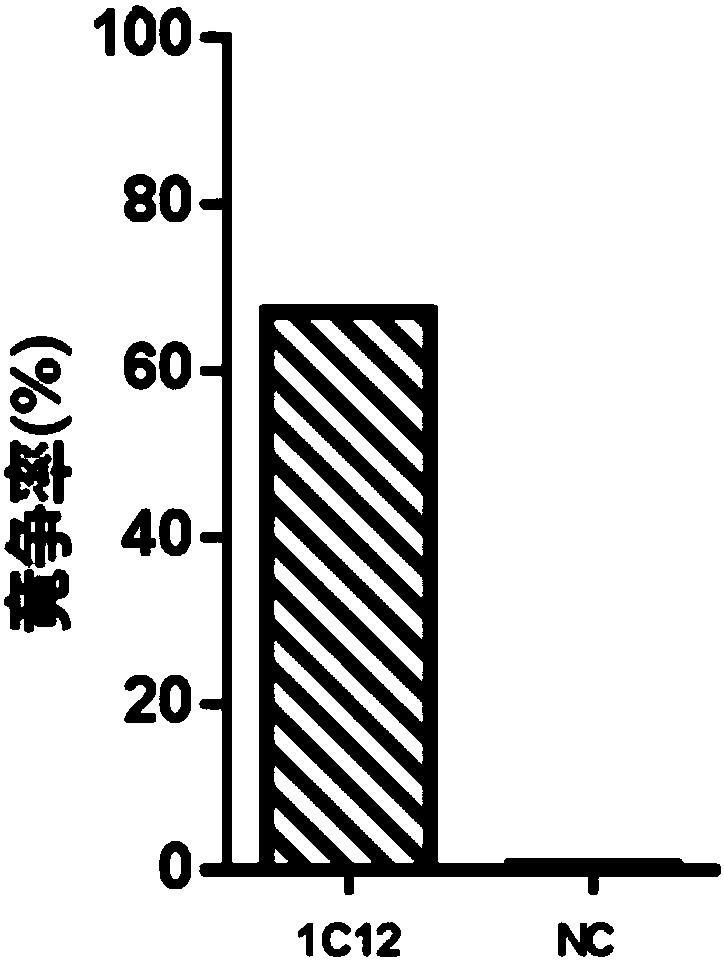
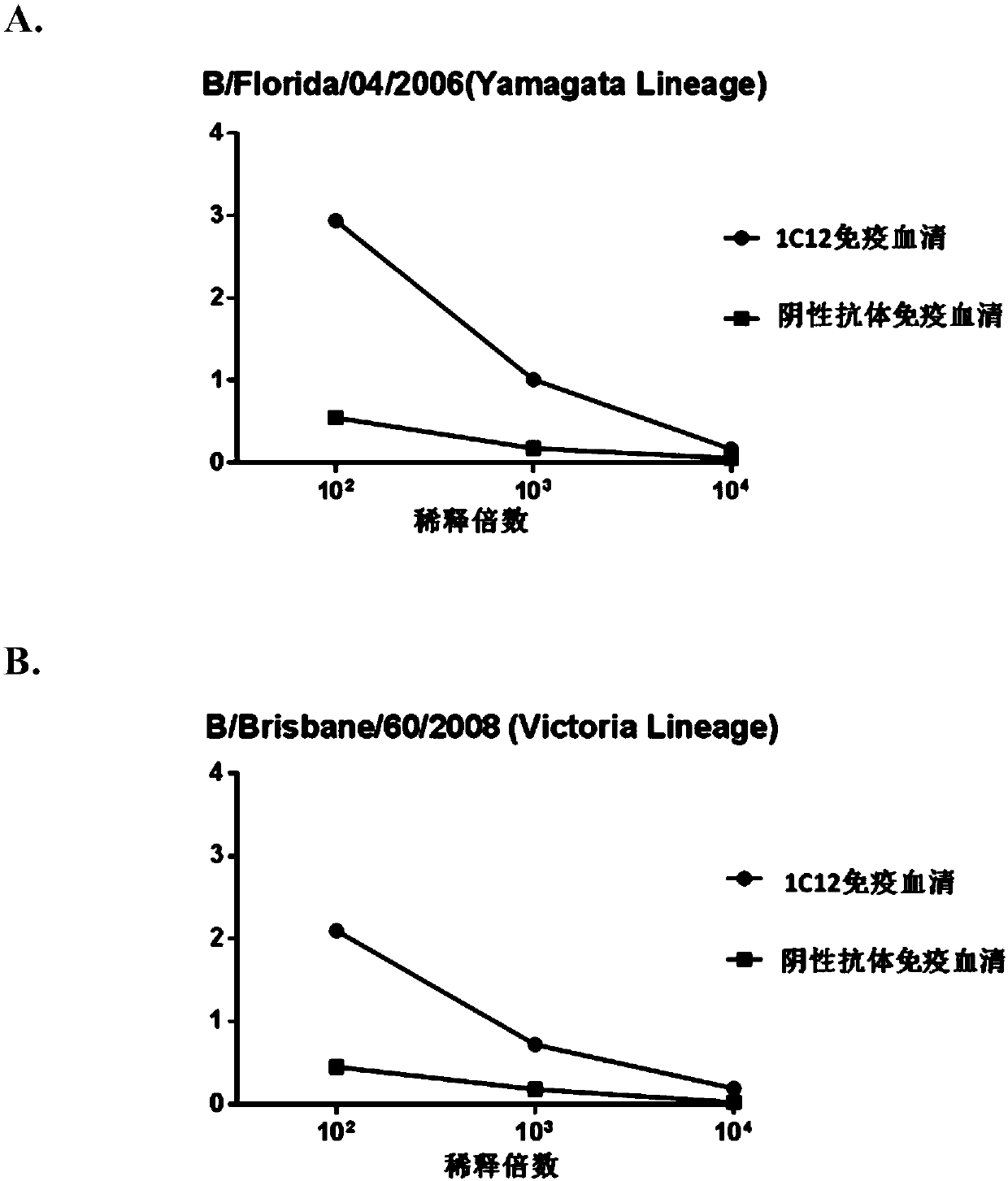
[0145] Example 3. Functional activity analysis of immune serum induced by anti-idiotypic antibody 1C12
[0146] Using representative strains of influenza B virus isolated from different times, different regions, representing different mutation types, and representative strains of influenza A virus used as a control, hemagglutination inhibition test (HI) and microneutralization test (MN) were used to evaluate Cross-reactivity of antisera elicited by antibody 1C12 to influenza B virus strains of different sublineages.
[0147] 1. Hemagglutination inhibition test (HI)
[0148] The hemagglutination inhibition test (HI) was carried out according to the WHO operation guideline. The experimental results are shown in Table 2. The results showed that the antiserum elicited by antibody 1C12 in mice reacted specifically with all tested influenza B strains except B / Lee / 1940, but not with tested influenza A viruses . This experimental result shows that the antiserum induced by antibody...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com