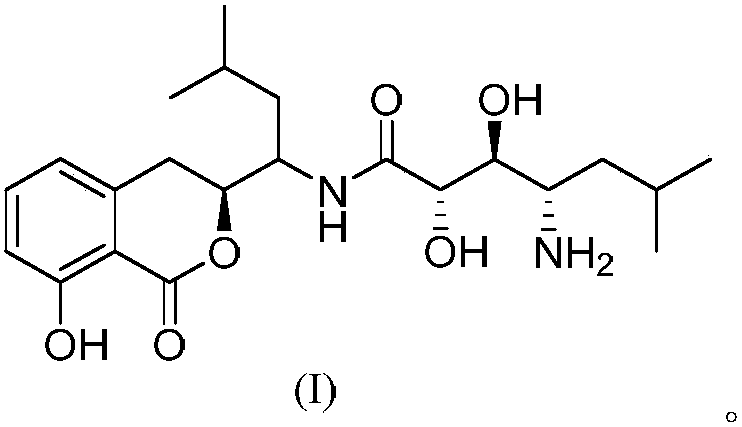
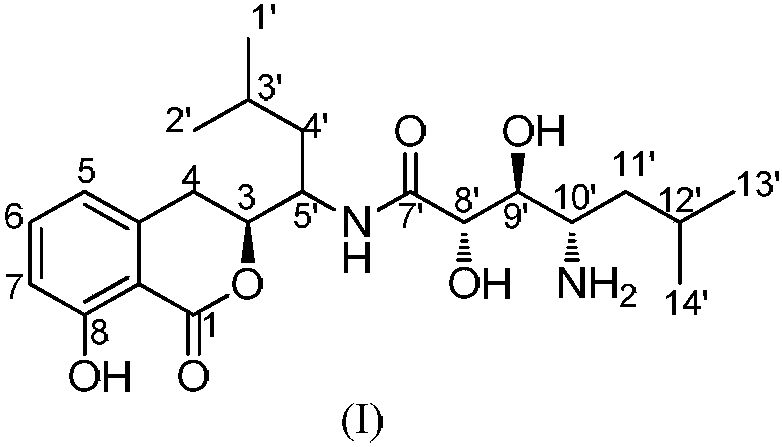
Preparation method for amicoumacin isocoumarin compound and application of compound
An isocoumarin and compound technology, which is applied in the field of pesticides and achieves the effects of high compound content, simple and efficient separation method, and good activity against crop pathogens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: Fermentative production and separation and purification of the compound of formula I
[0021] 1 Fermentation production
[0022] Fermentation culture of production bacteria: Paenibacillus sp.xy-2 is transferred from an inclined plane to a triangular flask equipped with a liquid medium after resuscitating (the composition of each bottle of medium: starch 1.0%, yeast extract 0.4%, peptone 0.2 %, add water to make up to 150mL), shake culture at 180rpm on a shaker at 28°C for 2-3 days, and use it as a seed culture solution. Then seed liquid is inoculated into the Erlenmeyer flask containing fresh, sterilized liquid culture medium (substrate composition as above) by 5~10% inoculum amount, under 28 ℃ 180rpm shaker culture 5 days, obtains fermentation culture fluid altogether About 30L.
[0023] 2 Obtaining of crude extract
[0024] The fermentation broth was centrifuged at 6000rpm to obtain mycelium and fermentation broth. Add HP20 macroporous resin (20g / L) ...
Embodiment 2
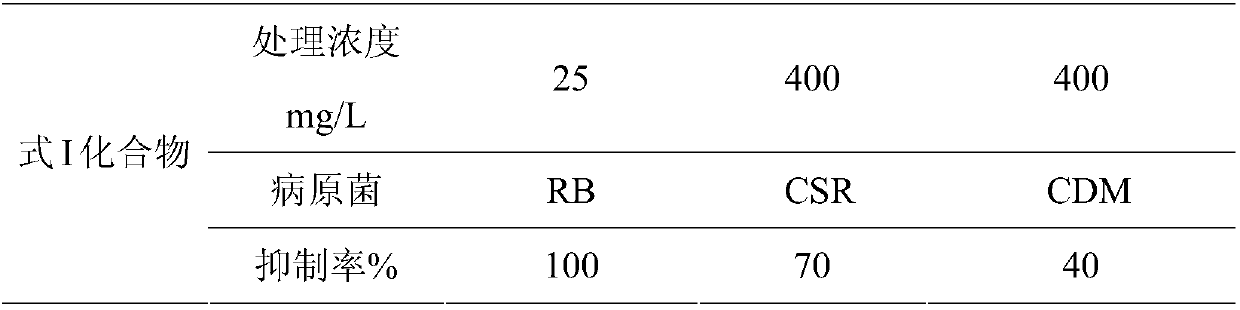
[0027] Embodiment 2: the antibacterial experiment of formula I compound to rice blast, corn stalk rust and cucumber downy mildew:
[0028] a. In vitro spore germination assay: the compound was dissolved in methanol as the test solution. Under sterile conditions, the test solution and rice blast fungus spore suspension were respectively added to a 96-well plate, and the 96-well plate was placed in a constant temperature incubator, and the inhibition of the compound on spore germination was detected after 24 hours. The inhibitory rate of the compound to the pathogenic bacteria is represented by 0-100%. The inhibitory rate of 0% means that the spore germination rate of the infection pathogenic bacteria group has no difference from that of the blank control group. The inhibitory rate of 100% means that the germination of the spores can be completely inhibited.
[0029] b. In vivo spray protection assay: Dissolve the compound in 2mL of acetone, add water containing 0.05% Tween-80 t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com