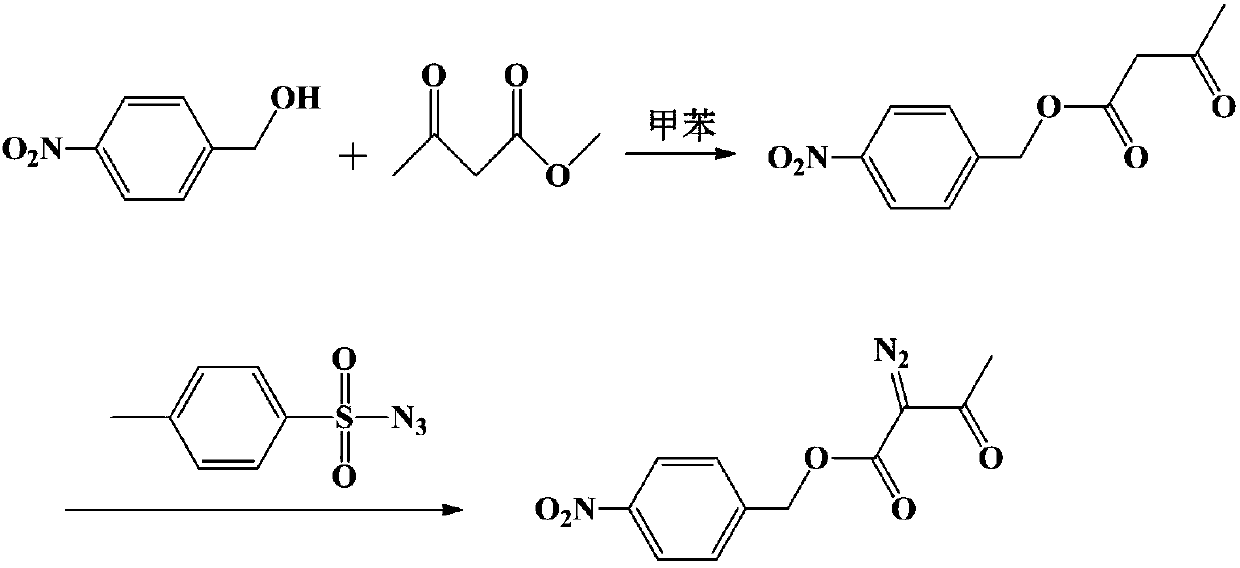
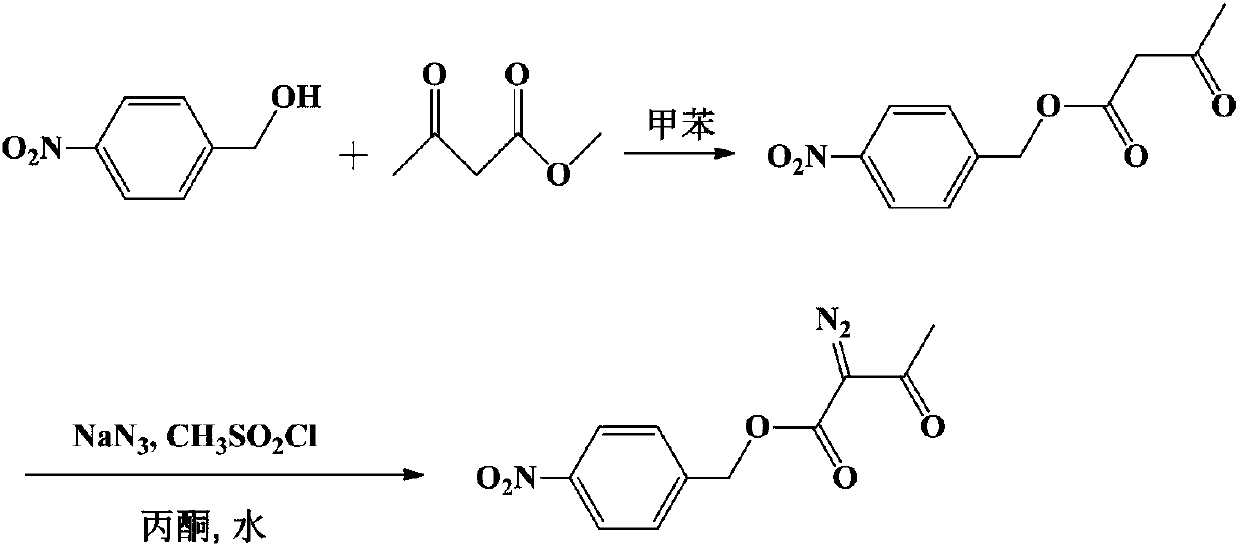
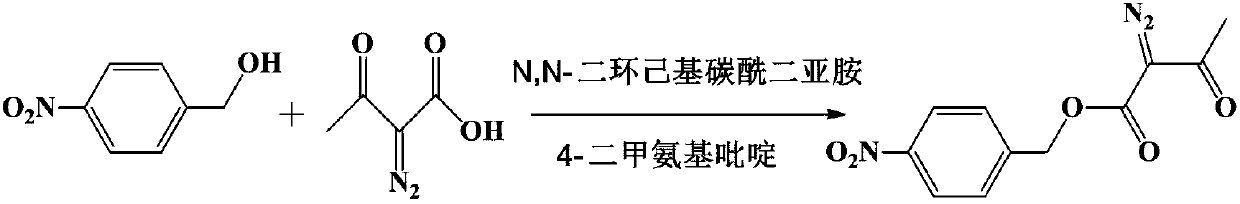
Method for preparing p-nitrobenzyl 2-diazoacetoacetate
A technology of ethyl diazo acetoacetate and diazo acetoacetic acid, applied in the field of fine chemical synthesis, can solve the problems of complex process operation, expensive catalyst, high nitrogen price, etc., achieve high purity, solve safety and occupational health problems, Simple to use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 275.7g (1.8mol) p-nitrobenzyl alcohol, 337.3g (2.16mol) ethyl 2-diazoacetoacetate, 2.8g sodium ethylate and 1380g toluene in the 3000ml reaction bottle with fractionation device, be warming up to reflux, Slowly collect ethanol fractions, react for 5.5 hours, drop to 0-10°C, keep stirring for 1 hour, filter, and dry to obtain 460g of 2-diazoacetoacetic acid p-nitrobenzyl ester product, yield 97.1%, HPLC: 99.8% .
Embodiment 2
[0032] Add 30.6g (0.2mol) p-nitrobenzyl alcohol, 46.8g (0.3mol) ethyl 2-diazoacetoacetate, 0.3g concentrated sulfuric acid and 245g xylene in a 500ml reaction flask with a fractionation device, and heat up to reflux , slowly collect the ethanol fraction, after reacting for 4 hours, lower the temperature to 20-30°C, keep stirring for 30 minutes, filter, and dry to obtain 50.8g of p-nitrobenzyl 2-diazoacetoacetate product, the yield is 96.5%, HPLC: 99.8 %.
Embodiment 3
[0034] Add 38.3g (0.25mol) p-nitrobenzyl alcohol, 46.8g (0.3mol) ethyl 2-diazoacetoacetate, 0.8g p-toluenesulfonic acid and 230g toluene in the 500ml reaction bottle that has fractionation device, be warming up to Reflux, slowly collect the ethanol fraction, react for 6 hours, drop to 10-20°C, keep stirring for 1 hour, filter, and dry to obtain 62.8g of p-nitrobenzyl 2-diazoacetoacetate, yield 95.5%, HPLC: 99.2 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com