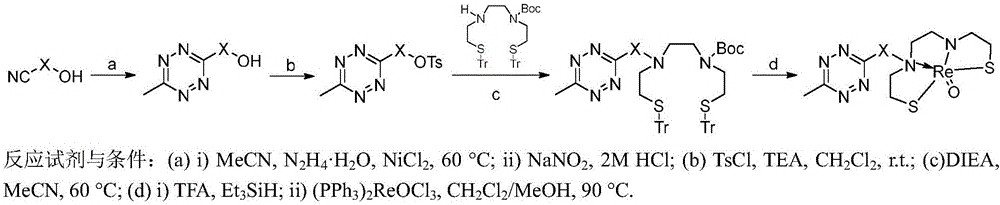
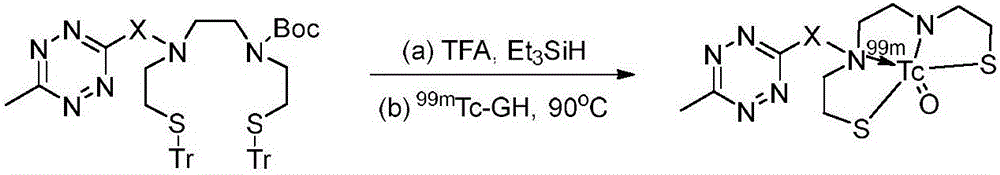
1,2,4,5-tetrazine compound for bioorthogonal reaction as well as preparation method and application thereof
A bioorthogonal, compound-based technique for rapid brain clearance in the fields of radiopharmaceutical chemistry and clinical nuclear medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Synthesis of Intermediate 1
[0033] Weigh 665.8mg 4-(hydroxymethyl)benzonitrile, 1026.3mg acetonitrile, 259.2mg anhydrous nickel chloride in a 250mL round bottom flask, add 7821.9mg hydrazine hydrate (concentration 80%) dropwise, and heat at 60°C for 24 hours, cooled to normal temperature, weighed 5002.5 mg of sodium nitrite, dissolved in water and added dropwise. Then place it in an ice-water bath, add 2M hydrochloric acid dropwise until there are no more bubbles, extract with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, concentrate by rotary evaporation, and separate on a silica gel column to obtain the product, namely intermediate 1, structure As follows, yield: 44%. 1 H NMR (400MHz, CDCl 3 )δ8.58(d, J=7.9Hz, 2H), 7.59(d, J=8.0Hz, 2H), 4.84(s, 2H), 3.10(s, 3H).
[0034]
Embodiment 2
[0035] Embodiment 2 synthetic intermediate 2
[0036] Intermediate 2 is prepared by reacting 3-(2-hydroxyethoxy)propionitrile with acetonitrile and hydrazine hydrate. The raw material ratio, solvent, and reaction conditions of the reaction are the same as those of Intermediate 1. The structure is as follows, and the yield : 17%. 1 H NMR (400MHz, CDCl 3 )δ4.11(t,J=6.2Hz,2H),3.69–3.67(m,2H),3.63–3.60(m,2H),3.58(t,J=6.2Hz,2H),3.05(s,3H ),2.56(s,1H).
[0037]
Embodiment 3
[0038] Embodiment 3 synthetic intermediate 3
[0039] Intermediate 3 is prepared by reacting 4-(2-hydroxyethoxy)benzonitrile with acetonitrile and hydrazine hydrate. The raw material ratio, solvent, and reaction conditions of the reaction are the same as those of Intermediate 1. The structure is as follows, and the yield : 44%. 1 H NMR (400MHz, CDCl 3 )δ8.55(d, J=8.9Hz, 2H), 7.10(d, J=8.9Hz, 2H), 4.20(t, J=4.6Hz, 2H), 4.03(t, J=4.6Hz, 2H) ,3.06(s,3H).
[0040]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com