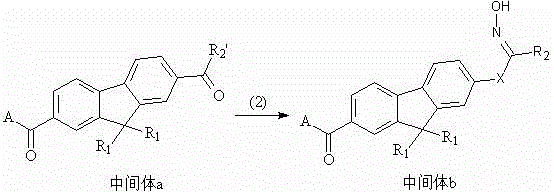
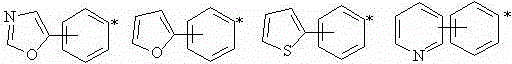Fluorine-containing oxime ester photoinitiator
A photoinitiator and ester technology, applied in the field of organic chemistry, can solve problems such as insufficient solubility, poor surface dryness, and easy yellowing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061]
[0062] (1) Preparation of intermediate 1a
[0063] Add 83g of raw material 1a, 134g of aluminum trichloride, and 100mL of dichloromethane into a 1000mL four-necked flask, drop the mixed solution of 73g of raw material 1b and 50mL of dichloromethane in an ice-water bath, and control the temperature below 10°C. After about 2 hours of dropping, continue stirring for 2 hours, then continue to add dropwise the mixed solution of 122g raw material 1c and 100mL of dichloromethane, about 2 hours after dropping, continue stirring to room temperature after dropping, and follow the liquid phase to complete the reaction. Then slowly pour the material into the dilute hydrochloric acid prepared by 800g ice water and 200mL concentrated hydrochloric acid (37%), stir while adding, and then pour it into the separatory funnel, separate the lower dichloromethane layer, and use 50mL dichloromethane Continue to wash the water layer, combine the dichloromethane layers, wash the dichlorome...
Embodiment 2
[0072]
[0073] (1) Preparation of intermediate 1a'
[0074] Add 97g of raw material 2a, 134g of aluminum trichloride, and 200mL of dichloromethane into a 1000mL four-necked flask, drop the mixed solution of 160g of raw material 2b and 100mL of dichloromethane in an ice-water bath, and control the temperature below 10°C. About 2h after the dropwise addition, continue to stir for 2h after the dropwise addition, and follow the liquid phase to complete the reaction. Then slowly pour the material into dilute hydrochloric acid made of 800g ice water and 300mL concentrated hydrochloric acid (37%), stir while adding, and then pour it into a separatory funnel, separate the lower dichloromethane layer, and wash it with 50mL dichloromethane Continue to wash the water layer, combine the dichloromethane layers, wash the dichloromethane layer with 5% aqueous sodium bicarbonate solution (300 mL each time, 3 times in total), then wash the dichloromethane layer with water until the pH is n...
Embodiment 3
[0083] Referring to the methods of Examples 1 and 2, compounds 3-18 shown in Table 1 below were synthesized using corresponding raw materials.
[0084] Table 1
[0085]
[0086] performance evaluation
[0087] 1. Solubility test
[0088] The solubility of the photoinitiator in PGMEA is one of the index parameters representing its solubility and measuring the application performance of the photoinitiator. The compounds of the formula (I) of the present invention and the existing photoinitiators containing fluorene oxime esters were selected to test their solubility in PGMEA at 25°C, and the results are shown in Table 2.
[0089] Table 2
[0090]
[0091]
[0092] As can be seen from the above table, the solubility of the compounds of the present invention and the compounds A and B as a comparison in PGMEA all meet the requirement that the solubility needs to be greater than 8% by weight in industrial applications, but the solubility of the compounds of the present...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com