A supported zirconium vanadate catalyst for the direct oxidation of benzene by oxygen to prepare phenol and its preparation method
A zirconium vanadate and catalyst technology, which is applied in the field of preparation of new catalysts, can solve the problems of many reaction steps, low yield, and no reports, and achieve the effects of simple preparation method, high catalytic activity and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 0.354g of ammonium metavanadate into 30ml of DMF, stir for 3h, and impregnate it at 25°C to obtain a mixed solution A. 0.529g of ZrCl 4 Dissolve in 20ml of DMF, and add the solution dropwise to the mixed solution A, and continue to stir for 5h, then wash the obtained mixture with DMF until the filtrate is colorless and transparent, then dry to obtain zirconium vanadate.
Embodiment 2
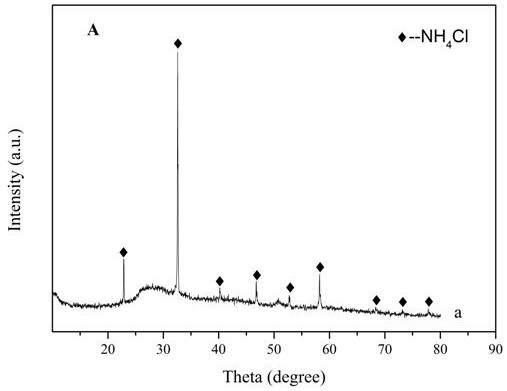
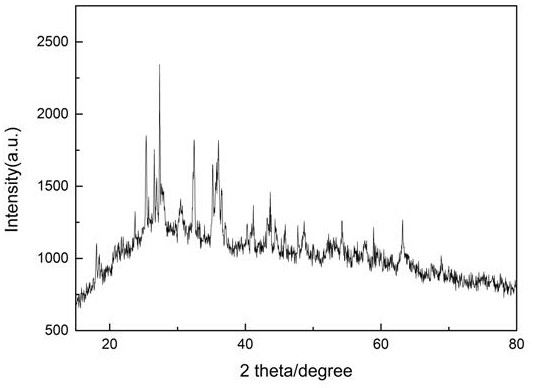
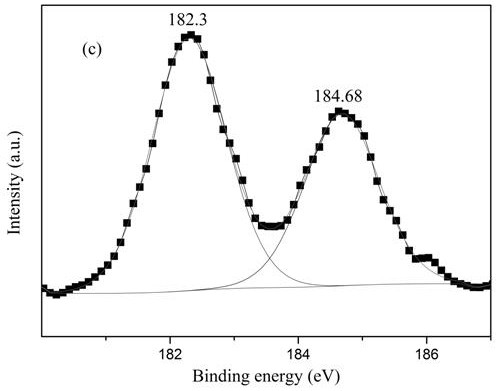
[0021] Add 5g of activated carbon and 0.354g of ammonium metavanadate to 30ml of DMF, stir for 6h, and impregnate it at 25°C, add 0.529g of ZrCl 4 Dissolve in 20ml of DMF, and add the solution dropwise to the mixed solution in step 1 and continue to stir for 18 hours, then wash the obtained mixture with DMF until the filtrate is colorless and transparent, then dry to obtain the activated carbon-supported zirconium vanadate catalyst . From figure 2 Can see NH 4 The presence of Cl peaks, indicating that ZrCl 4 and NH 4 VO 3 The reaction produces NH 4 Cl. The broad peak at 26-30° indicates that the catalyst is in an amorphous structure. The V-Zr complex also exists in an amorphous structure. Therefore, we can assume that ZrCl 4 and NH 4 VO 3 Amorphous vanadium-zirconium compounds were formed by co-precipitation. XPS analysis can infer the chemical valence states of Zr and V before and after the reaction. Depend on image 3 It can be seen that Zr 3d 5 / 2 and Zr 3d ...
Embodiment 3
[0024] 5g MWCNTs and 0.354g ammonium metavanadate were added to 30ml of DMF, stirred for 4h, and impregnated at 25°C, and 0.529g of ZrCl 4 Dissolve in 20ml of DMF, and add the solution dropwise to the mixed solution in step 1 and continue to stir for 18 hours, then wash the obtained mixture with DMF until the filtrate is colorless and transparent, then dry to obtain MWCNTs supported zirconium vanadate catalyst .
[0025] The prepared catalyst was used for benzene hydroxylation reaction, 150mg catalyst, 1mL benzene (0.84g), 3mL acetonitrile, 0.7g ascorbic acid were added to react in a 10mL autoclave, and 3MPa O 2 , reacted under magnetic stirring, the reaction temperature was 80°C, kept for 5h, and the yield of phenol was 8.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com