Mycoplasma hyopneumoniae, vaccine composition and application thereof
A technology of Mycoplasma hyopneumoniae and a vaccine composition, applied in the biological field, can solve the problems of increased vaccine production cost, slow growth, extremely high requirements, etc., and achieves the effects of good immunogenicity, high culture titer, and reduced usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0015] As an embodiment of the present invention, the culture of the Mycoplasma hyopneumoniae GZ strain of the present invention is a culture of passage 1 to 42.
[0016] The term "vaccine composition" used in the present invention refers to a pharmaceutical composition containing the immunogenicity of Mycoplasma hyopneumoniae, which can induce, stimulate or enhance the immune response of pigs against Mycoplasma pneumoniae. The vaccine composition comprises immune dose of attenuated live vaccine, inactivated vaccine, subunit vaccine or synthetic peptide vaccine of Mycoplasma hyopneumoniae strain.
[0017] As used herein, the term "inactivated vaccine", also called inactivated vaccine, refers to a suspension of inactivated virus used as an antigen to generate immunity. Examples of inactivated vaccines include whole virus vaccines and split vaccines. Inactivated vaccines can be readily produced using known methods. For example, whole virus inactivated vaccines can be obtained ...
Embodiment 1
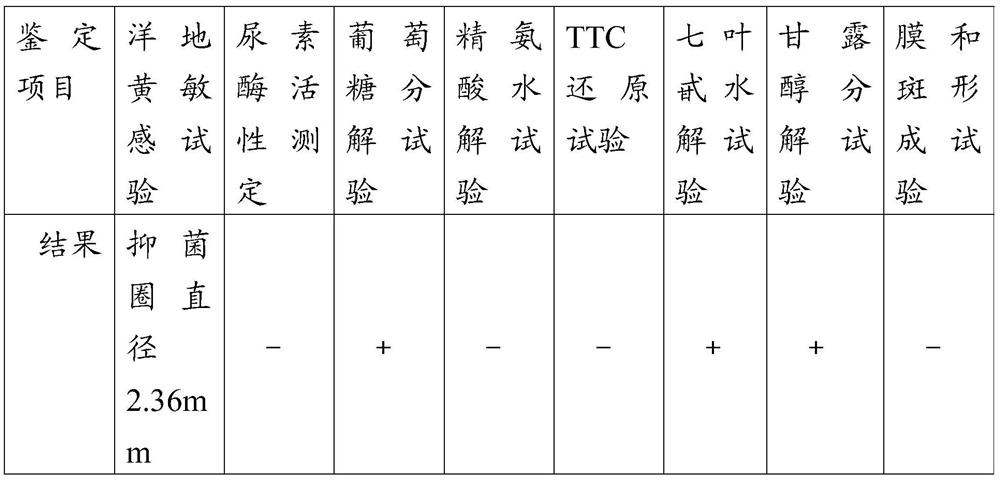
[0050] Example 1 Isolation and Identification of Mycoplasma Hyopneumoniae GZ Strain
[0051] 1. Source of disease material
[0052] The disease materials came from 20 lungs suspected to be caused by mycoplasma swine pneumonia collected from a pig farm in Henan Province in 2016.
[0053] 2. Multiplex PCR identification of disease materials
[0054] 2.1 Primer design and synthesis
[0055] Multiplex PCR (Mycoplasma hyopneumoniae, Mycoplasma suis and Mycoplasma hyorhina) primer reference (T.stakenbory et al. A multiplex PCR to Identify Porcine Mycoplasma Present in Broth Cultures) was synthesized:
[0056] mhp-f: 5'-TTCAAAGGAGCCTTCAAGCTTC-3';
[0057] mhr-f: 5'-GGGAAGAAAAAAATTAGGTAGGG-3';
[0058] mfl-f: 5'-CGGGATGTAGCAATACATTCAG-3';
[0059] m-r: 5'-AGAGGCATGATGATTTGACGTC-3';
[0060] It is estimated that the lengths of the amplified fragments of Mycoplasma hyopneumoniae, Mycoplasma hyorhinosus and Mycoplasma suis are 1000bp, 1129bp and 754bp respectively.
[0061] 2.2 DN...
Embodiment 2
[0084] The preparation of embodiment 2 mycoplasma pneumoniae GZ strain inactivated vaccine of swine
[0085] 1. Preparation of strains for production
[0086] After the freeze-dried strain of Mycoplasma hyopneumoniae GZ strain is unsealed, inoculate the liquid medium with 10% inoculum amount, cultivate at 37°C for 3-5 days, harvest when the pH value drops to 6.8-7.0, and use it as a first-class production seed after pure inspection . Take the first-grade seeds and inoculate the liquid medium with 5% inoculum amount, cultivate them at 37°C for 3-5 days, harvest when the pH value drops to 6.8-7.0, pass the pure inspection, and use them as the second-grade production seeds.
[0087] Liquid medium formula: beef heart extract (BD company) 300ml, double distilled water 360ml, correct the pH value to 7.4, sterilize at 121°C for 15 minutes, then add the following filter sterilization components: Hank's balanced salt solution (10×) 40ml, 0.25 (W / V) phenol red 10ml, horse serum 200ml,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com