Three-gene co-expression vector for synthesizing tetrahydropyrimidine and application of co-expression vector
A technology of co-expression vector and ectoine, which is applied in the field of enzyme engineering and compound biosynthesis, can solve the problem of low yield and achieve high synthesis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
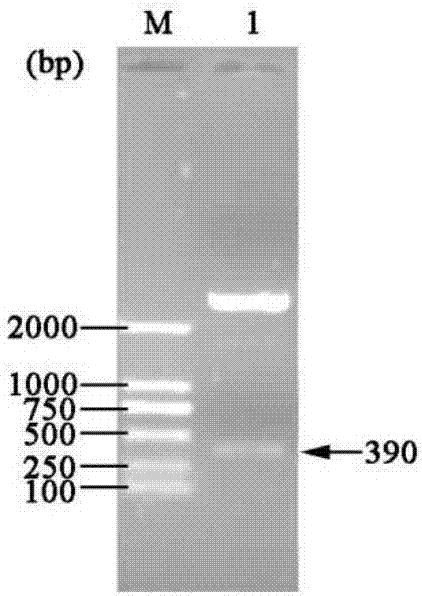
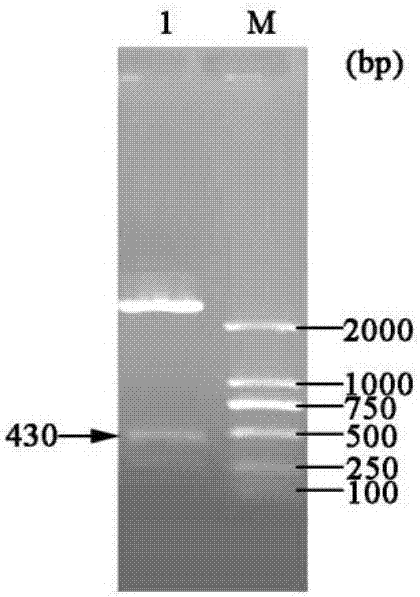
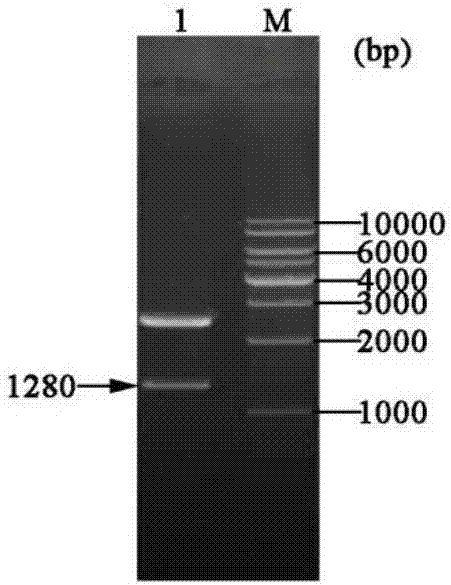
[0048] Example 1 Construction of three gene co-expression vectors
[0049] (1) Transformation vector pET-22b(+)
[0050] (a) Primer design: According to the nucleotide sequence of the region to be mutated near the T7 promoter and terminator in the commercial vector pET-22b(+), design PCR amplification reaction primers:
[0051] Nhe-F01: 5'-GAGATCTCGATGCTAGCAAATTAATACGACTC-3';
[0052] Spe-F01: 5'-AGGAGGAACTAGTTCCGGATTGGC-3';
[0053] Spe-R01: 5'-GCCAATCCGGAACTAGTTCCTCCT-3';
[0054] (b) Increase the SpeI recognition site: use site-directed mutagenesis (Site-Directed Mutagenesis), use the plasmid pET-22b(+) as a template, and use Spe-F01 and Spe-R01 as primers to perform site-directed mutagenesis PCR, PCR reaction conditions Pre-denaturation at 94°C for 4 min; denaturation at 94°C for 35 sec, annealing at 55°C for 1 min, extension at 72°C for 7 min, and 16 cycles; full extension at 72°C for 10 min.
[0055] The PCR product was digested with DpnI and transformed into Escheri...
Embodiment 2
[0071] Embodiment 2 biosynthetic ectoine
[0072] (1) Co-expression of three genes
[0073] The three-gene co-expression vector pET-22bNS-EctA / B / C was transformed into Escherichia coli BL21(DE3) competent cells, and the transformants were selected and cultured overnight in LB medium containing 100 μg / mL ampicillin at 37°C; The culture solution was inoculated into 100 mL of LB culture solution containing 100 μg / mL ampicillin at a ratio of 1:100, cultured with shaking at 37 °C and 180 rpm until the OD600 was 0.5-0.6, and then induced by adding IPTG with a final concentration of 1.0 mM at 28 °C for 15 h. Collect the bacteria by centrifugation at 8000rmp, wash the bacteria with 0.8% NaCl solution;
[0074] (2) Whole-cell catalytic synthesis of ectoine
[0075] Weigh 1g of bacteria and resuspend in 20mL of reaction solution (50mM PBS buffer, pH 7.5; 150mM L-Asparticacid, 100mM Glycerol), culture at 40°C and 200rmp for 3 hours, then centrifuge to remove the bacteria, and use HPLC ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com