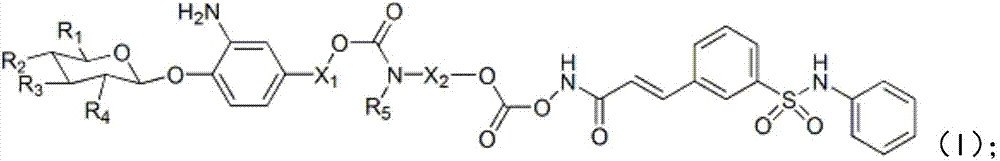
Belinostat derivative based on formic acid, and preparation method and applications thereof
A belistat and derivative technology, applied in the field of formic acid-based belistat derivatives and their preparation, to achieve the effects of solving drug side effects, controlling production costs, and improving water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
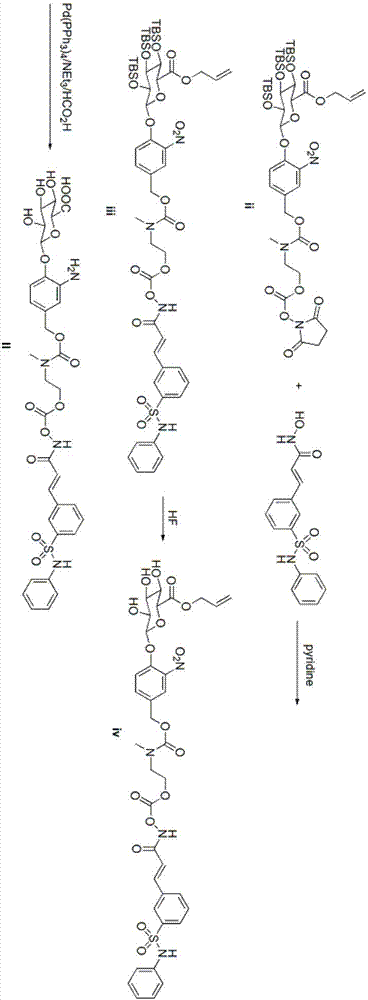
[0068] Example 1 O-{[N-methyl-N-4-(2,3,4-tri-O-tert-butyldimethylsilyl-6-allyl-β-D-glucopyranose Preparation of aldehyde acid-1-yl)-3-nitrobenzyloxycarbonyl]-2-aminoethyl}-formyl-belinostat (iii)
[0069] Bellinostat (469mg, 1.47mmol) was dissolved in 6.4mL of tetrahydrofuran, and the system was cooled to 0°C; then 1.6mL of pyridine was slowly added dropwise; the mixture was first stirred at 25°C for 5 minutes, and then compound ii (1.3 g, 1.34 mmol). The reaction solution was kept at 25°C and stirred for 16 hours. Afterwards, it was diluted with 20 mL of water and quenched, extracted with ethyl acetate (20 mL×2); the combined organic layers were washed with 20 mL of saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated with a rotary evaporator. The residue was separated by preparative chromatography (petroleum ether: ethyl acetate = 3:1to1:1) to obtain the following white solid iii (1.035g, 65.9%);
[0070] Characterization of the product: LCMS: ...
Embodiment 2
[0071] Example 2 O-{[N-methyl-N-4-(6-allyl-β-D--pyranoglucuronic acid-1-yl)-3-nitrobenzyloxycarbonyl]-2- Preparation of aminoethyl}-formyl-belinostat (iv)
[0072] Compound iii (400mg, 0.34mmol) was dissolved in a mixed solvent of tetrahydrofuran (20mL) and acetonitrile (20mL);
[0073] Hydrofluoric acid (4.8mL, 40% in H 2 O) Dissolve in acetonitrile (15.2mL) to prepare a solution, add the solution to the solution containing compound iii at 0°C; stir the reaction solution at 20°C for 2 days, then concentrate the reaction solution to about 8mL , Preparative HPLC gave the following white solid iv (130 mg, 30.7%).
[0074] Characterization of the product: 1 H NMR(400MHz,DMSO-d6):δ12.39(brs,1H),10.35(brs,1H),7.99(s,1H),7.89-7.86(m,2H),7.74(d,J=7.6Hz ,1H),7.68-7.58(m,3H),7.46(d,J=8.8Hz,1H),7.23(t,J=7.6Hz,2H),7.09(d,J=7.6Hz,2H),7.03 (t,J=7.6Hz,1H),6.60(d,J=16.0Hz,1H),5.93-5.85(m,1H),5.56-5.51(m,2H),5.34-5.28(m,3H), 5.18(d, J=10.8Hz, 1H), 5.08(s, 2H), 4.61(d, J=4.0Hz, 2H), 4.36...
Embodiment 3
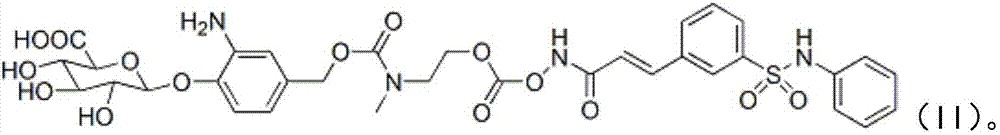
[0076] Example 3 O-{[N-methyl-N-4-(β-D-pyranoglucuronic acid-1-yl)-3-aminobenzyloxycarbonyl]-2-aminoethyl}-formyl - Preparation of Belinostat (II)
[0077] Compound iv (183mg, 0.22mmol) was dissolved in 12mL THF;
[0078] 95 μL of triethylamine and 7 μL of formic acid were dissolved in 190 μL of tetrahydrofuran, and the solution was added dropwise to the solution of compound iv in tetrahydrofuran;
[0079] Pass argon for 10 minutes, add a little tetrakistriphenylphosphopalladium, stir at room temperature for about 30 minutes until the raw material disappears, concentrate with a rotary evaporator, and separate the residue by preparative chromatography (acetonitrile: water = 20:1) to obtain the white target product II (134mg, 80%);
[0080] Characterization of the product: 1 H NMR (400MHz, DMSO-d 6 ):δ12.59(brs,1H),10.41(brs,1H),7.93-7.89(m,1H),7.66(s,1H),7.80-7.78(m,1H),7.51-7.49(m,1H ),7.45(d,J=16.0Hz,1H),7.16(m,2H),7.05-7.02(m,1H),6.97-6.95(m,5H),6.92(d,J=16.0Hz,1H) ,6....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com