Manufacture of a triiodinated contrast agent
A technology of contrast agent and iodination, which is applied in the production field of tri-iodinated contrast agent, can solve problems such as being unfavorable to the environment, uneconomical and the like, and achieve the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
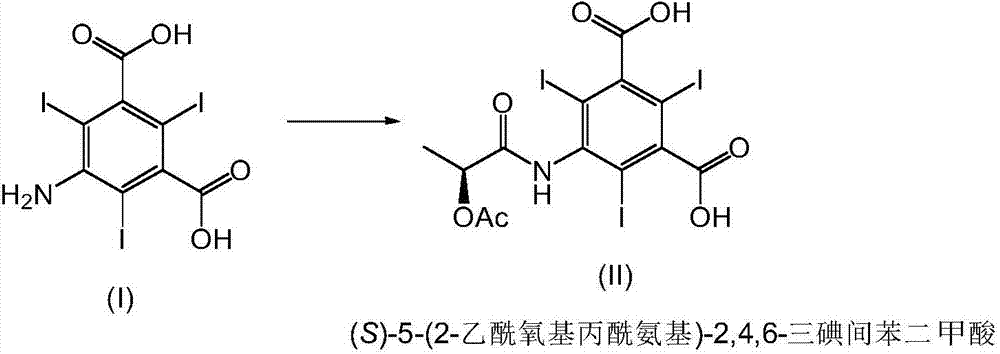
[0050] Preparation of (S)-5-(2-acetoxypropionylamino)-2,4,6-triiodoisophthalic acid
[0051] To a solution of 5-amino-2,4,6-triiodoisophthalic acid (1.0 g, 1.79 mmol) in DMA (5 ml) was added dropwise (S)-1-chloro-1-oxopropane-2- Glycolacetate (0.75ml, 5.91mmol). The resulting mixture was stirred at about 50°C for 5 hours and 20 minutes. 80 ml of water are added to the reaction mixture at room temperature, after which the resulting suspension is cooled to a temperature between 0° C. and 5° C. and stirred at this temperature for 25 minutes. The suspension was filtered and the solid was washed with water. The product was dried in a vacuum oven at 40°C to obtain (S)-5-(2-acetoxypropionylamino)-2,4,6-triiodoisophthalic acid (0.695g, 1.03mmol). MS, 1 H-NMR and 13 C-NMR data is consistent with the structure of (S)-5-(2-acetoxypropionylamino)-2,4,6-triiodoisophthalic acid.
[0052] Yield: 57.5%
[0053] HPLC purity: 99.91%
[0054] (Chromatographic column: μPorasil 125A 10 μm ...
Embodiment 2
[0058] Preparation of (S)-5-(2-acetoxypropionylamino)-2,4,6-triiodoisophthalic acid
[0059] To a suspension of 5-amino-2,4,6-triiodoisophthalic acid (50.0 g, 89.5 mmol) in DMA (100 ml) was slowly added (S)-1 at a temperature between 25 °C and 29 °C -Chloro-1-oxopropan-2-yl acetate (37.4ml, 295.4mmol). The resulting mixture was heated to about 50°C and stirred at this temperature for about 8 hours, after which time the heat was removed and the mixture was stirred at room temperature for about 14 hours. The reaction mixture was slowly added to water (500ml) at a temperature between 22°C and 30°C with vigorous stirring. After the addition, 300 ml of water were added to the suspension. The suspension was stirred for a further 5 hours at about 22°C, after which the white solid was filtered off and washed twice (30 ml each) with water previously cooled at about 5°C. The product was dried in a vacuum oven at about 50°C to obtain (S)-5-(2-acetoxypropionylamino)-2,4,6-triiodoisopht...
Embodiment 3
[0065] Preparation of (S)-1-((3,5-bis(chlorocarbonyl)-2,4,6-triiodophenyl)amino)-1-oxopropan-2-yl acetate
[0066] Phosphorus pentachloride (37.1g, 178.3mmol) was added in batches to (S)-5-(2-acetoxypropionylamino)-2,4,6-triiodoisophthalic acid obtained in Example 2 Formic acid (40.0 g, 59.4 mmol) in DMA (200 ml). The reaction mixture was stirred at about 40°C for 6 hours, after which it was added dropwise over 1 hour to water (400 ml) cooled to a temperature between 0°C and 5°C with vigorous stirring. The resulting suspension was stirred for a further hour at a temperature between 0°C and 5°C and the white precipitate was filtered off. The white solid was washed with water (80ml) previously cooled to a temperature between 0°C and 5°C. The solid was resuspended in a mixture of water (103ml) and isopropanol (80ml) and stirred at this temperature for 15 minutes. The suspension was warmed to a temperature between 20°C and 25°C and stirred at this temperature for 30 minutes, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com