Drug for treating cardiovascular and cerebrovascular diseases, preparation method and application of drug
A technology for cardiovascular and cerebrovascular diseases and drugs, applied in cardiovascular system diseases, drug combinations, pharmaceutical formulations, etc., can solve the problem of no other activity or efficacy, and achieve the effect of enhancing hypoxia tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
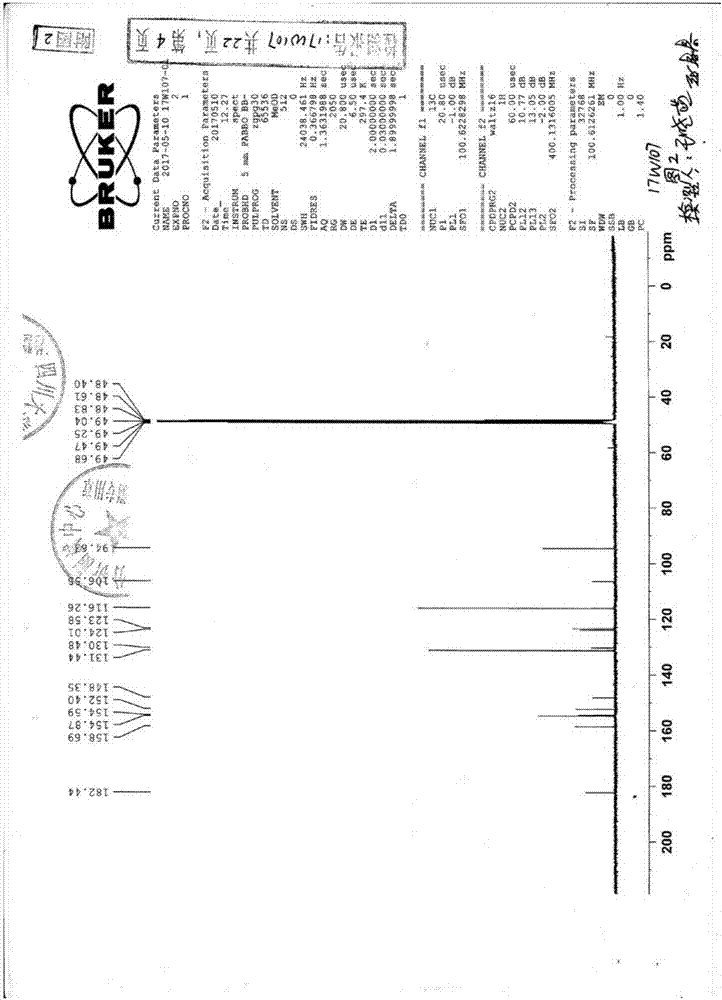
[0025] Example 1 Preparation of 6-hydroxygenistein by Shegan aglycon
[0026] Add Shegan aglycon into the reaction kettle, then add 5 times the amount (ml / g) of 95% ethanol (V / W), stir with a mixer and heat to reflux, and then add 3 times the amount (ml / g) after the Shegan aglycon is completely dissolved. g) Hydroiodic acid (content ≥ 45% V / W), stirred, heated to reflux for 5 to 24 hours, using silica gel GF 254 TLC inspection to determine the end point of the reaction (developing solvent is chloroform: methanol: formic acid 10:1:0.1). Release the reaction solution, leave it at room temperature overnight, and precipitate pale yellow needle-like crystals, filter and wash with water until neutral to obtain crude 6-hydroxygenistein; use recrystallization to obtain refined 6-hydroxygenistein with a yield of 85%.
[0027] The product obtained in this embodiment is pale yellow needle crystal; odorless and tasteless. Soluble in methanol, ethanol, almost insoluble in water. Melting...
Embodiment 2
[0030] Example 2 Preparation of 6-hydroxygenistein by Sheganoside
[0031] Put Shegan glycosides into the reaction kettle, add 5 times the amount (ml / g) of 95% ethanol (V / W), stir with a mixer and heat to reflux, and then add 3 times the amount (ml / g) when it becomes a uniform paste Hydroiodic acid (content ≥ 45%) (V / W), stirred, heated to reflux for 5 to 24 hours, using silica gel GF 254 Thin-layer chromatography inspection to determine the reaction end point (developing solvent is chloroform: methanol: formic acid 10:1:0.1), release the reaction solution, leave it at room temperature overnight, precipitate off-white precipitate, filter, wash with water until neutral, and obtain 6-hydroxy dye Crude lignin; 6-hydroxygenistein was obtained by recrystallization method, with a yield of 70%.
[0032] The chemical reaction equation is as follows:
[0033]
Embodiment 3
[0034] Example 3 Preparation of 6-hydroxygenistein from puereticin
[0035] Put puerarin into the reaction kettle, then add 5 times the amount (ml / g) of 95% ethanol (V / W), stir with a mixer and heat to reflux, and then add 3 times the amount ( ml / g) of hydroiodic acid (content ≥ 45%) (V / W), stir, heat and reflux for 5 to 24 hours, use silica gel GF 254 Thin-layer chromatography was used to determine the end point of the reaction (the developer was chloroform:methanol:formic acid 10:1:0.1), the reaction liquid was released, and left overnight at room temperature, and light yellow needle-like crystals were precipitated, filtered, washed with water until neutral, and 6 -Crude hydroxygenistein; 6-hydroxygenistein was obtained by recrystallization method, with a yield of 80%.
[0036] The chemical reaction equation is as follows:
[0037]
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com