Multiple PCR primer group and kit for detecting gene related to colorectal cancer administration
A colorectal cancer, gene detection technology, applied in recombinant DNA technology, biochemical equipment and methods, microbial determination/inspection, etc. The effect of starting volume reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Design and synthesis of multiplex PCR primers for colorectal cancer individualized drug gene mutation detection
[0039] Use the primer design software whose patent application title is a multiplex PCR primer and its design method for amplifying BRCA1 / 2 gene (application number is 201610737156.7) to design primers, and input the genomic region (bed file format, per The line content is "chromosome-tab-start coordinate-tab-end coordinate"), and the output is multiple pairs of primers designed to be amplified in one reaction. The specific implementation ideas are as follows: 1) Use Primer3 software to design as many available primers as possible for each target region as alternatives; 2) According to the specificity of primer amplification, whether the primer contains simple repeat sequences, and whether the primer contains High-frequency SNP sites (defined as sites with a minor allele frequency of more than five thousandths in dbSNP) and other conditions filter ...
Embodiment 2
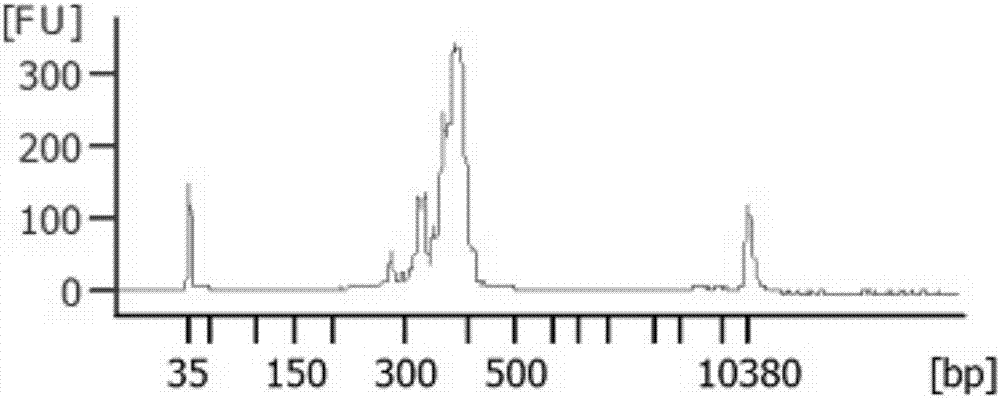
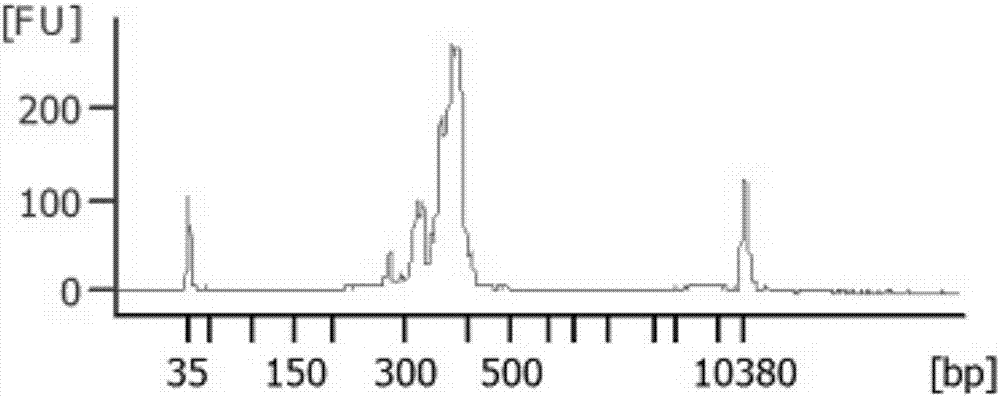
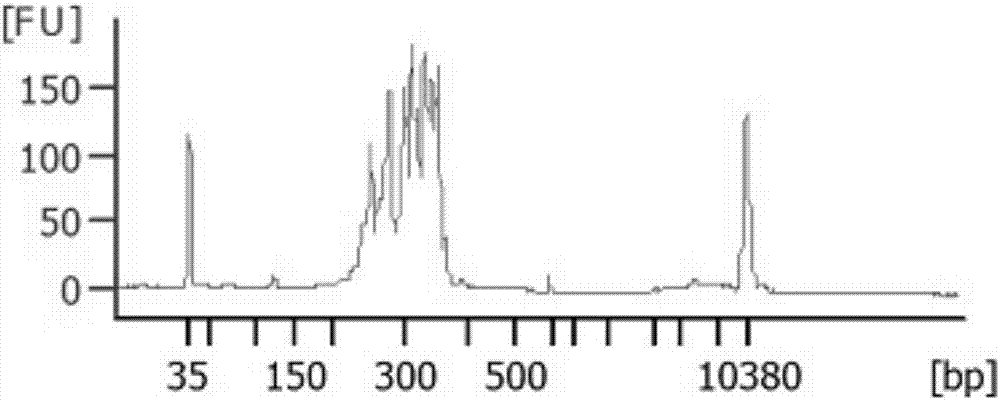
[0043] Example 2 Different types of sample library construction and mutation detection
[0044] 1. Experimental materials
[0045] The samples involved in this test are: genomic DNA of normal human blood, genomic DNA of MCF10A cell line, and FFPE genomic DNA of colorectal cancer. Genomic DNA of various types of samples was extracted using DNA extraction kits.
[0046] Genomic DNA extraction: use Qiagen DNeasy Blood&Tissue Kit, and follow the kit instructions to extract genomic DNA. The obtained genomic DNA is subjected to gel electrophoresis and quality detection using NanoDrop100. It is required that the genomic DNA has no obvious degradation, the concentration is greater than 20ng / μL, A260 / A280>1.8, A260 / A230>2.0, and Qubit 3.0 is used for accurate quantification.
[0047] A detection kit for colorectal cancer drug-related genes, including a primer set in Example 1, a universal downstream primer (URS), and a linker primer for Illuminate sequencer sequencing library construc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com