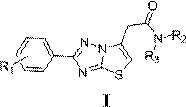
Thiazolotriazole-6-acetamide derivatives and their application
A thiazolo, triazole technology, applied in the field of medicine, can solve problems such as drug resistance or pharmacokinetic defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Preparation of 2-{2-(4-chlorophenyl)thiazolo[3,2-b][1,2,4]triazol-6-yl}acetic acid
[0018] Add 0.1 mol of thiosemicarbazide and 100 mL of dichloromethane into a 250 mL three-necked flask, stir in an ice-water bath to dissolve, and then add 0.13 mol of pyridine. Slowly add 0.13 mol of 4-chlorobenzoyl chloride dropwise at 0-5°C, the dropwise addition is completed in 20 minutes, react at 15°C for 2 hours, and end the reaction. A large amount of white solid appeared in the system and was filtered. Dissolve the obtained white solid in 80 mL of 5% sodium hydroxide solution by mass fraction, heat to reflux for 2 hours, cool down to room temperature, adjust the pH to 5-6 with dilute hydrochloric acid with a mass fraction of 3.65%, a large amount of light yellow solid precipitates, filter , recrystallized to obtain 18.3 g of 5-(4-chlorophenyl)-3-mercapto-1,2,4-triazole, yield 86.7%, ESI-MS (m / z): 212.3 (M+H )+.
[0019] Add 0.6mol of 5-(4-chlorophenyl)-3-mercapto-1,2,4-triaz...
Embodiment 2
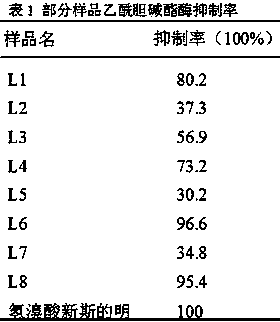
[0023] N,N-Dimethyl-2-{2-(4-chlorophenyl)thiazolo[3,2-b][1,2,4]triazol-6-yl}-acetamide (L1) preparation of
[0024] Add 10mmol dimethylamine hydrochloride, 10mmol 2-{2-(4-chlorophenyl)thiazolo[3,2-b][1,2,4]triazol-6-yl}acetic acid, and 20mL dichloromethane In the round bottom flask, then add 12mmolEDCI, 12mmolHOBt and triethylamine 10mmol, after room temperature reaction 12h, reaction solution is successively washed with 5%HCl, 5%NaHCO3, saturated NaCl solution, after drying, column chromatography separates to obtain white solid, Yield 32%.
[0025] 1H-NMR (300MHz, DMSO), δ(ppm): 7.50 (2H, d, J = 8.4Hz), 7.41 (1H, s), 7.38 (2H, d, J = 8.4Hz), 4.89 (2H, s ), 3.96 (3H, s), 3.02 (3H, s); ESI-MS (m / z): 321.4 (M+H)+.
Embodiment 3
[0027] N,N-Diethyl-2-{2-(4-chlorophenyl)thiazolo[3,2-b][1,2,4]triazol-6-yl}-acetamide (L2) preparation of
[0028] Dimethylamine hydrochloride was replaced with diethylamine, and the synthesis method was referred to Example 2, and the yield was 34%.
[0029] 1H-NMR (300MHz, DMSO), δ(ppm): 7.52 (2H, d, J = 9.0Hz), 7.42 (1H, s), 7.37 (2H, d, J = 9.0Hz), 4.86 (2H, s ), 3.98 (2H, m), 3.32 (2H, m), 1.18 (3H, t), 1.05 (3H, t); ESI-MS (m / z): 349.0 (M+H)+.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com