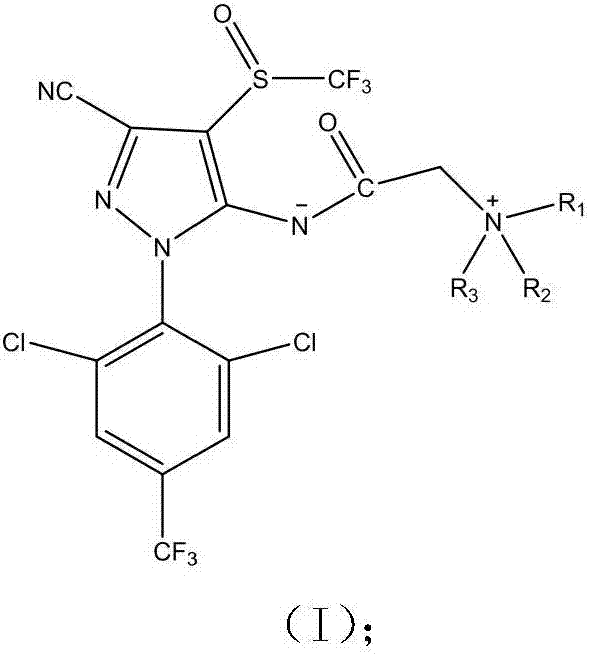
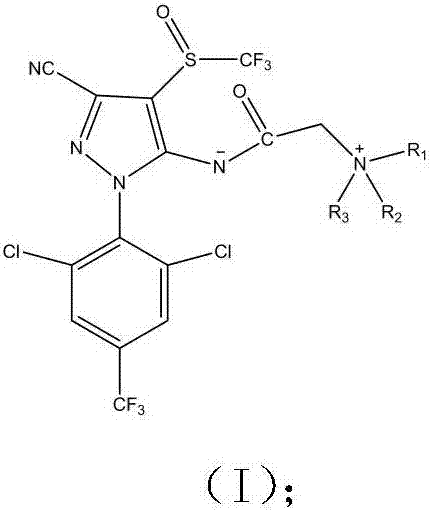
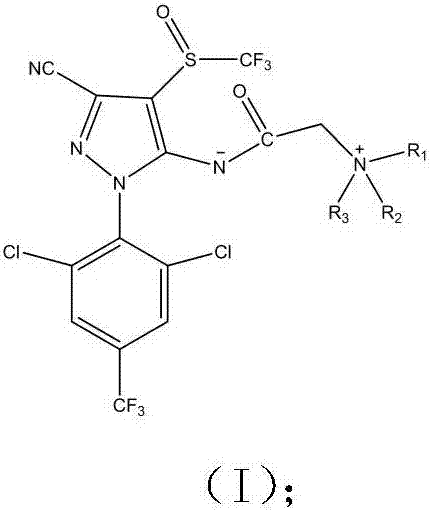
Phenylpyrazole zwitterionic compound and application thereof in control of resistant pests
A phenylpyrazole, zwitterion technology, applied in the application, pesticide, organic chemistry and other directions, can solve the problems such as the ineffectiveness of resistant pests, increase water solubility, etc., to increase the spectrum of pest control and overcome resistance , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1 phenylpyrazole zwitterionic compound
[0037] Preparation method: Add 4.40g (0.01mol) of fipronil to a 100mL three-necked flask equipped with a magnetic stirrer, a drying tube and a dropping funnel, add 40mL of dry DMF, stir to dissolve, and then add 2.76g of anhydrous potassium carbonate ( 0.02mol), add 1.78mL 98% bromoacetyl bromide dropwise, stir at room temperature for 30min, then add quinoline (0.012mol) dissolved in DMF, stir overnight at room temperature until the reaction is complete, quench with ice water, extract with ethyl acetate , the organic phase was dried with anhydrous sodium sulfate, and the filtrate was filtered, then subjected to column chromatography after vacuum distillation, and recrystallized from methanol / petroleum ether to obtain yellow crystals with a yield of 62.40%, m.p.103-105°C. The detection data is:
[0038] 1 H NMR (600MHz, DMSO) δ9.24 (dd, J = 5.8, 1.3Hz, 1H, Ar-H), 8.96 (d, J = 8.3Hz, 1H, Ar-H), 8.23 ...
Embodiment 2
[0044] Preparation of embodiment 2 phenylpyrazole zwitterionic compounds
[0045] The preparation method is the same as in Example 1, wherein the molar ratio of fipronil: bromoacetyl bromide: 5-aminoquinoline: anhydrous potassium carbonate is 1:1.1:1.2:1.8. Recrystallization from methanol / petroleum ether gave red crystals with a yield of 45.6%, m.p.258-265°C. The detection data is:
[0046] 1H NMR (600MHz, DMSO) δ9.11(d, J=8.5Hz, 1H), 9.02–8.98(m, 1H), 7.87(d, J=1.4Hz, 1H, Ar-H), 7.80(d, J=1.5Hz, 1H, Ar-H), 7.70(t, J=8.3Hz, 1H), 7.56(dd, J=8.5, 5.8Hz, 1H), 7.00(s, 2H, NH 2 ),6.97(d,J=8.7Hz,1H),6.91(d,J=8.0Hz,1H),5.30(dd,J=35.7,16.3Hz,2H,CH 2 ).
[0047] 13 C NMR(151MHz,DMSO)δ168.59,151.86,149.43,148.86,141.63,139.97,137.51,137.27,135.32,135.15,132.06,125.92,125.29,123.54,121.76,119.01,117.05,113.20,110.09,103.93,102.16,99.97 ,62.10.
[0048] HRMS(ESI-TOF):Extract mass calculated for C 23 h 12 Cl 2 f 6 N 6 o 2 S requires[M+H] + 621.0109, found 621.0096; [M+Na] + ...
Embodiment 3
[0052] Preparation of embodiment 3 phenylpyrazole zwitterionic compounds
[0053] The preparation method is the same as in Example 1, wherein the molar ratio of fipronil:bromoacetyl bromide:N,N-dimethyl-5-aminoquinoline:anhydrous potassium carbonate is 1:1.2:1.3:2.3. Recrystallization from methanol / petroleum ether gave red crystals with a yield of 15.52%, m.p.245-249°C. The detection data is:
[0054] 1 H NMR (600MHz, DMSO) δ9.15 (dd, J = 5.8, 1.3Hz, 1H), 8.94 (d, J = 8.5Hz, 1H), 7.93–7.89 (m, 1H), 7.85 (d, J = 1.4Hz, 1H, Ar-H), 7.80(d, J=1.5Hz, 1H, Ar-H), 7.77(dd, J=8.6, 5.7Hz, 1H), 7.52(d, J=8.9Hz, 1H ), 7.34(d, J=7.9Hz, 1H), 5.46(dd, J=34.5, 16.4Hz, 2H, CH 2 ),2.95(s,6H,CH 3 ,CH 3 ).
[0055] 13 C NMR(151MHz,DMSO)δ168.45,152.81,151.71,149.58,143.29,140.52,137.47,135.94,135.26,135.11,132.22,125.88,125.32,124.27,123.57,121.77,119.52,116.49,113.16,111.49,85.91,75.89 ,62.24,45.43.
[0056] HRMS(ESI-TOF):Extract mass calculated for C 25 h 16 Cl 2 f 6 N 6 o 2 S re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com