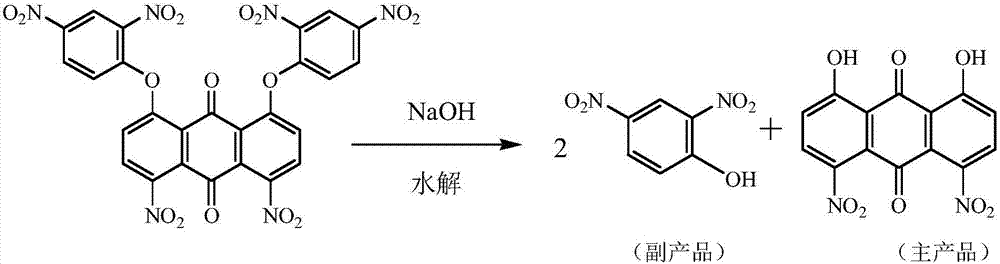
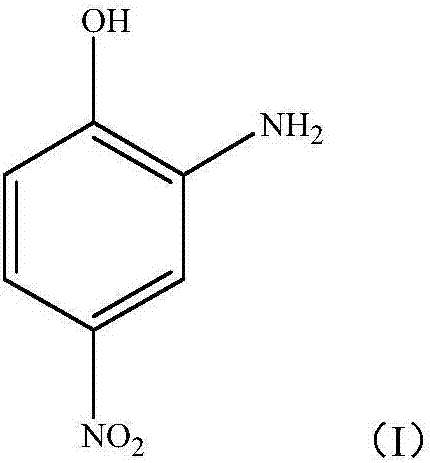
Method for preparing 2-amino-4-nitrophenol by using disperse blue 2BLN byproduct
A technology for nitrophenol and dinitrophenol is applied in the field of preparing 2-amino-4-nitrophenol, and can solve the problem of low content and purity of 2,4-dinitrophenol, reduction of recycling efficiency, value, pH Poor adjustment and other problems, to achieve the effect of high practical application value, reduce adverse effects, and reduce the trouble of processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Utilize the method for preparing 2-amino-4-nitrophenol by-product of disperse blue 2BLN, comprises the following steps:
[0061] A) adjust the pH of the mother liquor in the hydrolysis reaction step in the preparation process of disperse blue 2BLN to be 2.2, and filter to obtain the crude product of 2,4-dinitrophenol;
[0062] B) 35 g of the crude product obtained in step A) is beaten with water, and reduced with sodium hydrosulfide in a buffer system of ammonium chloride (the amount of ammonium chloride is prepared by dissolving 28 g of water), and the reaction temperature is controlled to be 85° C.;
[0063] C) Control the reaction end point of step B) by liquid chromatography, add sodium chloride for salting out after the end point is reached, cool to 5° C. and filter to obtain the crude product of 2-amino-4-nitrophenol;
[0064] D) dissolving the crude product obtained in step C) in water, raising the temperature to 81° C. and filtering while hot;
[0065] E) Acidi...
Embodiment 2
[0069] Utilize the method for preparing 2-amino-4-nitrophenol by-product of disperse blue 2BLN, comprises the following steps:
[0070] A) adjust the pH of the mother liquor in the hydrolysis reaction step in the preparation process of disperse blue 2BLN to be 3.0, and filter to obtain the crude product of 2,4-dinitrophenol;
[0071] B) 30 g of the crude product obtained in step A) is beaten with water, and reduced with sodium hydrosulfide in a buffer system of ammonium chloride (the amount of ammonium chloride is prepared by dissolving 7.5 g in water), and the reaction temperature is controlled to be 55° C. ;
[0072] C) Use liquid chromatography to control the reaction end point of step B), add sodium chloride to salt out after the end point is reached, cool to 8°C, and filter to obtain the crude product of 2-amino-4-nitrophenol;
[0073] D) dissolving the crude product obtained in step C) in water, raising the temperature to 65° C., and filtering while hot;
[0074] E) Ac...
Embodiment 3
[0078] Utilize the method for preparing 2-amino-4-nitrophenol by-product of disperse blue 2BLN, comprises the following steps:
[0079] A) adjust the pH of the mother liquor in the hydrolysis reaction step in the preparation process of disperse blue 2BLN to be 2.5, and filter to obtain the crude product of 2,4-dinitrophenol;
[0080] B) 18.5g of the crude product obtained in step A) is beaten with water, reduced with sodium hydrosulfide in the buffer system of ammonium chloride (the consumption of ammonium chloride is prepared by dissolving 5.55g in water), and the control reaction temperature is 60 ℃;
[0081] C) Control the reaction end point of step B) by liquid chromatography, add sodium chloride for salting out after the end point is reached, cool to 25° C. and filter to obtain the crude product of 2-amino-4-nitrophenol;
[0082] D) dissolving the crude product obtained in step C) in water, raising the temperature to 70° C. and filtering while hot;
[0083] E) Acidify t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com