Sample for verifying capacity of staphylococcus aureus in medicine and preparation method thereof
A staphylococcal, proficiency-proven technology, applied in biochemical equipment and methods, microorganism-based methods, bacteria, etc., to solve problems such as fatal, direct patient infection with Staphylococcus aureus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Samples for proficiency testing of Staphylococcus aureus in medicines, including target bacteria and background flora, the target bacteria is Staphylococcus aureus, and the background flora consists of Escherichia coli (E.coil), pneumonia Klebsiella (Klebsiellapnenmoniae), Bacillus cereus (Bacillus cereus) composition.
[0030] The sample is based on trehalose, skimmed milk powder and sterile water, wherein the volume fraction of trehalose is 12%, and the volume fraction of skimmed milk powder is 0.5%.
[0031] The target concentration of target bacteria in the sample is 10 2 CFU / mL, the target concentration of background flora is 10 3 CFU / mL.
Embodiment 2
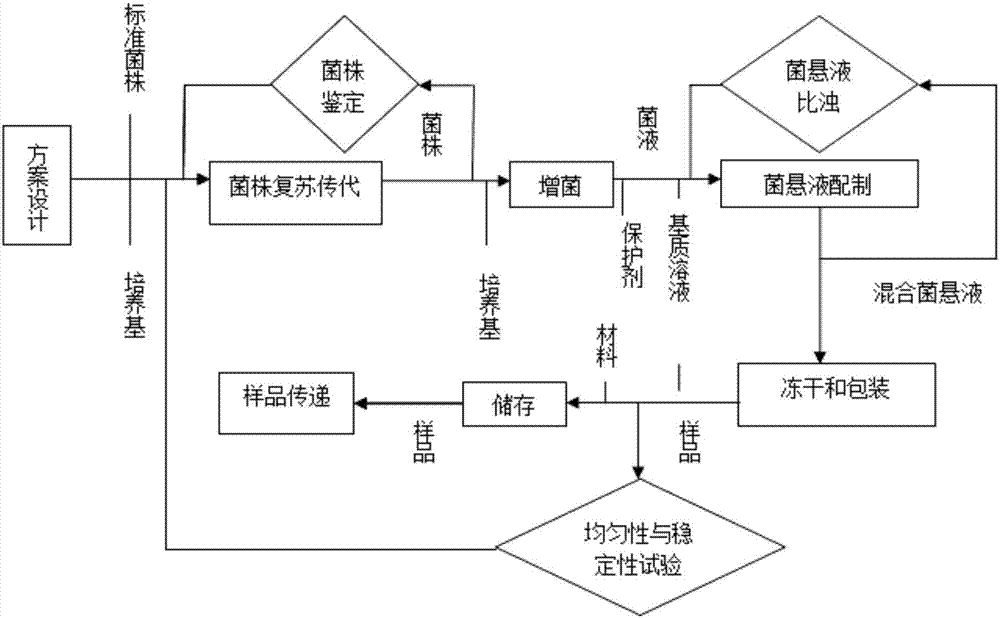
[0033] Such as figure 1 As shown, a preparation method for proficiency testing samples of Staphylococcus aureus in medicines in Example 1, including the selection of sample addition strains, preparation of lyoprotectant, sample freeze-drying, sample homogeneity and stability test four Steps, the specific steps are as follows:
[0034] 1. Selection of sample addition strains
[0035] According to target bacteria: Staphylococcus aureus (Staphylococcus aureus), background flora: Escherichia coli (E. The standard strains are all purchased from institutions designated by the government, and a strain certificate is attached to ensure the traceability of the strains.
[0036] 2. Preparation of lyoprotectant
[0037] The sample was prepared with trehalose, skimmed milk powder and sterilized water as the matrix (volume fraction: trehalose 12%, skimmed milk powder 0.5%) to prepare a lyoprotectant.
[0038] 3. Sample freeze-drying
[0039] (1) Strain recovery and passage
[0040] A...
Embodiment 3
[0070] Each step of the preparation method of the Staphylococcus aureus proficiency verification sample in the drug described in this embodiment is all the same as in Example 2, and the different technical parameters are: in the preparation of the bacterial suspension, the concentration of the target bacteria in the target bacterial suspension Concentration according to 5×10 2 CFU / mL preparation, the concentration of each background bacteria in the background flora suspension according to 10 3 CFU / mL preparation; freeze-drying process 45h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com