Combined application of ginsenoside and berberine or berberine salt to preparation of drugs for treating atherosclerosis or hyperlipidemia
A technology for atherosclerosis and ginsenosides, applied in the field of pharmacy, to achieve good application prospects, reduce expression, excellent atherosclerosis and hyperlipidemia effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
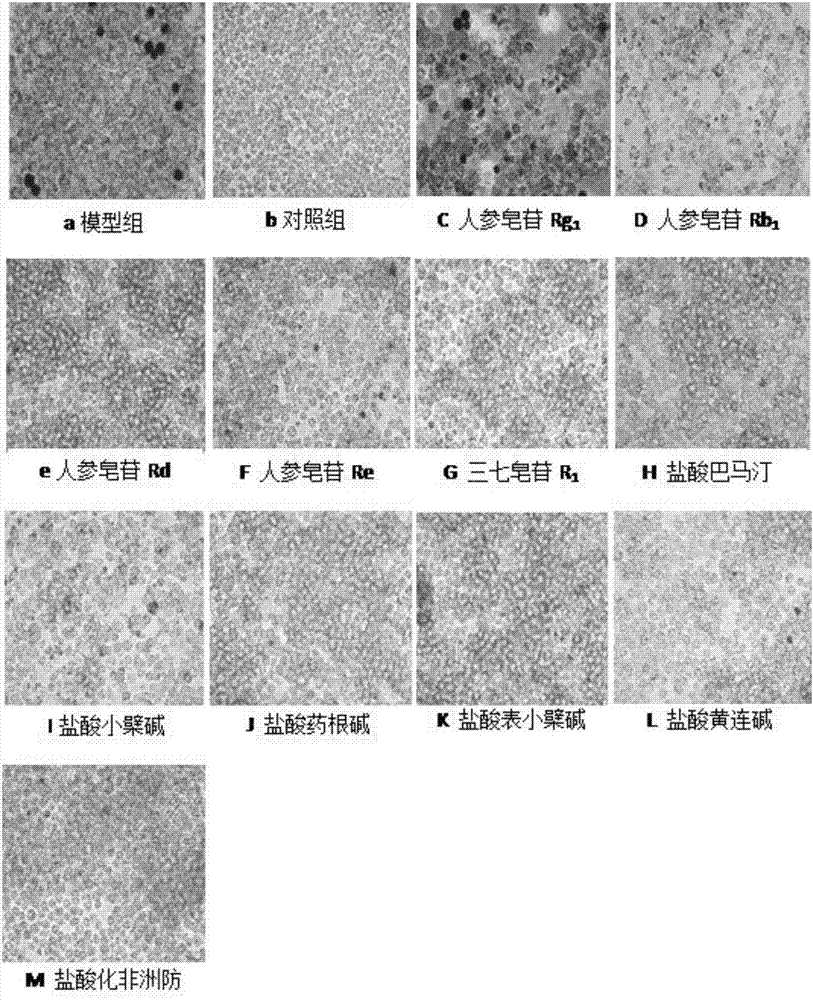
[0036] Example 1 Effect of ginsenoside Rb1 combined with berberine hydrochloride on the formation of RAW264.7 macrophage foam cells
[0037] 1.1 Cell culture and modeling
[0038] The cells used in the experiment were RAW264.7 macrophage cell line. Preparation of complete medium: high glucose (DMEM) medium containing 10% fetal bovine serum (FBS), 1% penicillin and streptomycin mixed solution. placed in 5% CO 2, cultured in a cell incubator at 37°C. When the cell fusion reached 75%, the cells were subcultured: 0.25% trypsin was digested for 1 mim, the adherent cells were blown gently, the cell suspension was transferred to a centrifuge tube, and centrifuged at 800r / min for 5min. Discard the supernatant, add complete cell culture medium to resuspend the cells, transfer them to a new culture dish with a pipette, and place in 5% CO 2 , cultured in a 37°C incubator.
[0039] Establish foam cell model: RAW264.7 macrophage cell line at 1×10 5 Inoculated on a 24-control plate an...
Embodiment 2
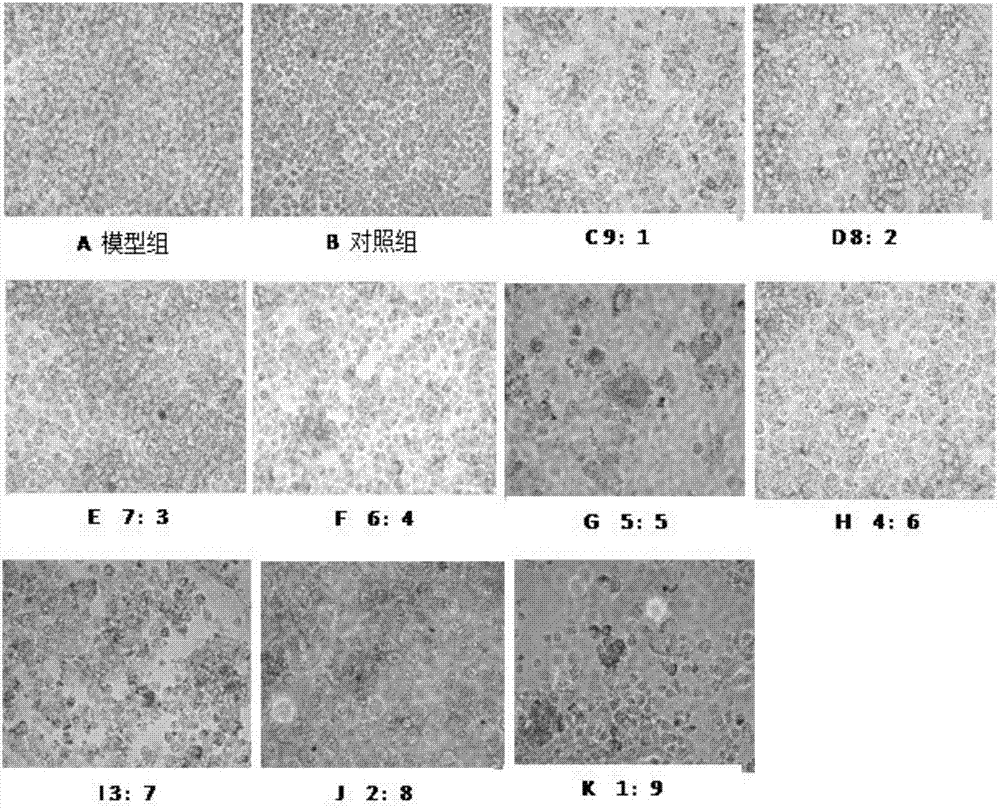
[0064] Example 2 Ginsenoside Rb 1 Effects of Combined Administration with Berberine Hydrochloride on Cholesterol Ester Content in RAW264.7 Macrophages
[0065] The culture medium was divided into 5 categories according to the experimental treatment requirements, corresponding to the corresponding cell culture groups: blank group (no FBS medium); model group, ginsenoside Rb 1 Combined with berberine hydrochloride (berberine hydrochloride: ginsenoside Rb 1 =9:1), ginsenoside Rb1 group, berberine hydrochloride. After incubation with complete medium containing 10% FBS for 5 days, the medium was removed, washed twice with PBS, fixed with 4% paraformaldehyde for 10 min, washed three times with PBS, and then the oil red O stock solution was mixed with pure water at a ratio of 3:2. Let stand for 10min. Filter on filter paper. The filtrate was added to the dish and stained in the dark for 5 minutes. Remove the Oil Red O working solution, add 60% isopropanol solution, wash once, wa...
Embodiment 3
[0067] Example 3 Ginsenoside Rb 1 Effects of combination with berberine hydrochloride on the viability of RAW264.7 macrophages
[0068] High glucose (DMEM) medium containing 10% fetal bovine serum (FBS), 1% penicillin and streptomycin mixed solution. placed in 5% CO 2 , cultured in a cell incubator at 37°C. When the cell fusion reached 75%, the cells were subcultured: 0.25% trypsin was digested for 1 mim, the adherent cells were blown gently, the cell suspension was transferred to a centrifuge tube, and centrifuged at 800r / min for 5min. Discard the supernatant, add complete cell culture medium to resuspend the cells, transfer them to a new culture dish with a pipette, and place in 5% CO 2 , cultured in a 37°C incubator.
[0069] RAW264.7 macrophage cell line with 1×10 5 Inoculated on a 24-control plate and incubated in a CO2 incubator at 37°C for 24 hours. After the cells grew well, they were incubated with complete medium containing 40% FBS for 5 days, and the medium was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com