Graphite phased carbon nitride nano-ring material and its preparation method
A graphite-phase carbon nitride and nano-ring technology, applied in chemical instruments and methods, nanotechnology, nitrogen compounds, etc., can solve the problems of reducing product quantum efficiency, photocatalytic effect, and poor electrical conductivity, and achieve excellent photogenerated electron-space Hole separation ability, reduced recombination probability, and uniform product size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
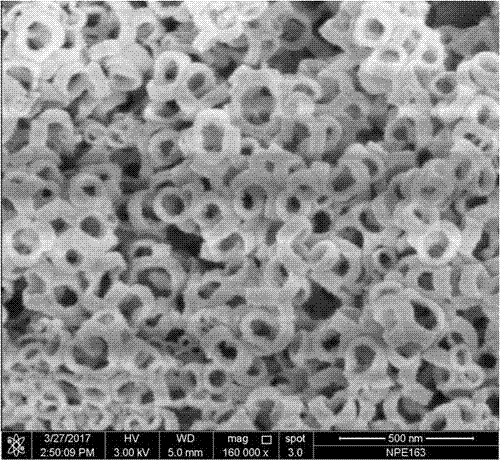
[0040] (1) Spread 10g of melamine powder on the bottom of quartz boat container 1, and spread 0.2g of silica nanospheres with an average diameter of 194nm on the bottom of quartz boat container 2, and place quartz boat container 1 and container 2 Inside the quartz tube.
[0041] (2) Place the quartz tube in (1) in a two-stage heating furnace, and the inner container 1 and container 2 of the quartz tube are located in the low-temperature section and the high-temperature section respectively; The container 1 filled with melamine is located upstream of the carrier gas, and the container 2 filled with silica nanospheres is located downstream of the carrier gas.
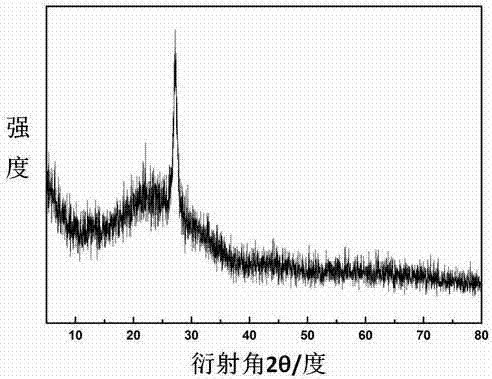
[0042] (3) The high-temperature section where the silica ball container 1 is placed is raised from room temperature to 550°C at a rate of 10°C / min, and the temperature of the low-temperature section where the melamine container 2 is placed is raised to 330°C at a rate of 10°C / min. The constant temperature reaction was ca...
Embodiment 2
[0046] (1) Spread 10g of melamine powder on the bottom of quartz boat container 1, and spread 0.2g of silica nanospheres with an average diameter of 194nm on the bottom of quartz boat container 2, and place quartz boat container 1 and container 2 Inside the quartz tube.
[0047] (2) Place the quartz tube in (1) in a two-stage heating furnace, and the inner container 1 and container 2 of the quartz tube are located in the low-temperature section and the high-temperature section respectively; The container 1 filled with melamine is located upstream of the carrier gas, and the container 2 filled with silica nanospheres is located downstream of the carrier gas.
[0048] (3) The high-temperature section where the silica ball container 1 is placed is raised from room temperature to 550°C at a rate of 10°C / min, and the temperature of the low-temperature section where the melamine container 2 is placed is raised to 330°C at a rate of 10°C / min. The reaction was carried out at constant...
Embodiment 3
[0052] (1) Spread 10g of melamine powder on the bottom of quartz boat container 1, and spread 0.2g of silica nanospheres with an average diameter of 535nm on the bottom of quartz boat container 2, and place quartz boat container 1 and container 2 in Inside the quartz tube.
[0053] (2) Place the quartz tube in (1) in a two-stage heating furnace, and the inner container 1 and container 2 of the quartz tube are located in the low-temperature section and the high-temperature section respectively; The container 1 filled with melamine is located upstream of the carrier gas, and the container 2 filled with silica nanospheres is located downstream of the carrier gas.
[0054] (3) The high-temperature section where the silica ball container 1 is placed is raised from room temperature to 550°C at a rate of 10°C / min, and the temperature of the low-temperature section where the melamine container 2 is placed is raised to 330°C at a rate of 10°C / min. The reaction was carried out at const...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com