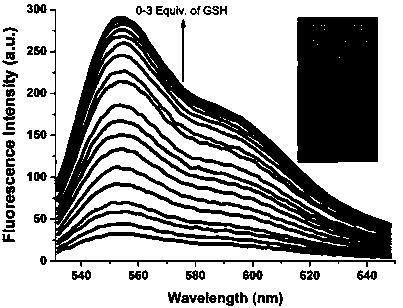
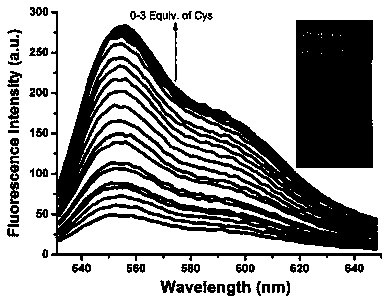
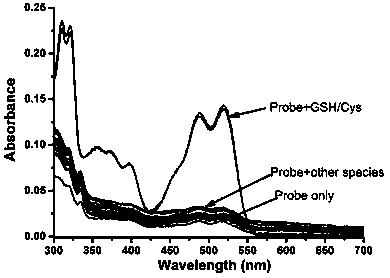
A kind of reactive mercapto compound probe and preparation method thereof
A sulfhydryl compound and compound technology, applied in chemical instruments and methods, organic chemistry, fluorescence/phosphorescence, etc., can solve the problems of small capillary diameter, affecting separation reproducibility, difficult operation, etc., and achieve the effect of significant enhancement of fluorescence intensity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] (1) Preparation of compound 1
[0047] Add 40 mL of toluene to a 150 mL round bottom flask, then add 11.0 g (0.1 mol) of resorcinol and 14.8 g (0.1 mol) of phthalic anhydride, stir to dissolve, stir and reflux for 5 hours, and gradually generate a solid, which is filtered off with suction The solid was recrystallized from methanol to obtain an orange solid.
[0048] The synthetic route is as follows:
[0049]
[0050] (2) Preparation of acenaphthol
[0051] Add 5.0g (32mmol) acenaphthene into a 100mL three-necked flask, and then add 6.5mL phosphorus oxychloride (POCl 3 ) Slowly add 13.5mL N,N-dimethylformamide (DMF) into the three-necked flask with a constant pressure funnel (the dripping is completed within 15 minutes), stir for 5 minutes and then slowly increase the temperature to 90~95℃, continue stirring 3 After hours, the reaction solution was obtained; the reaction solution was added to 270 mL of ice-water mixture, and a large amount of viscous solid appeared after stirr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com