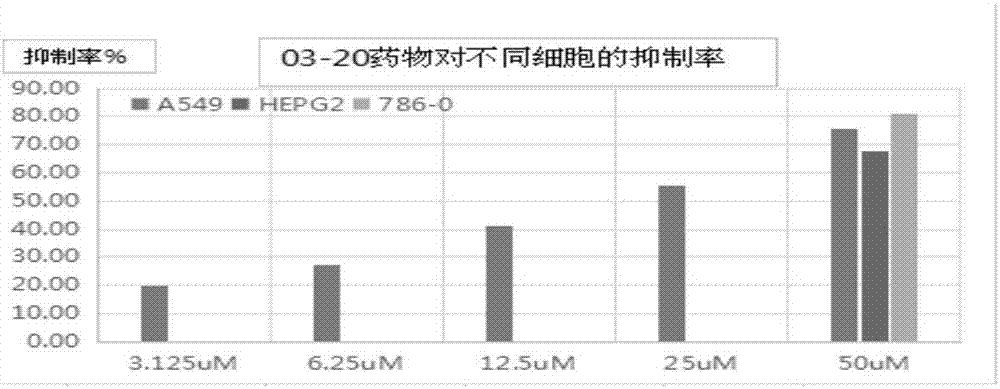
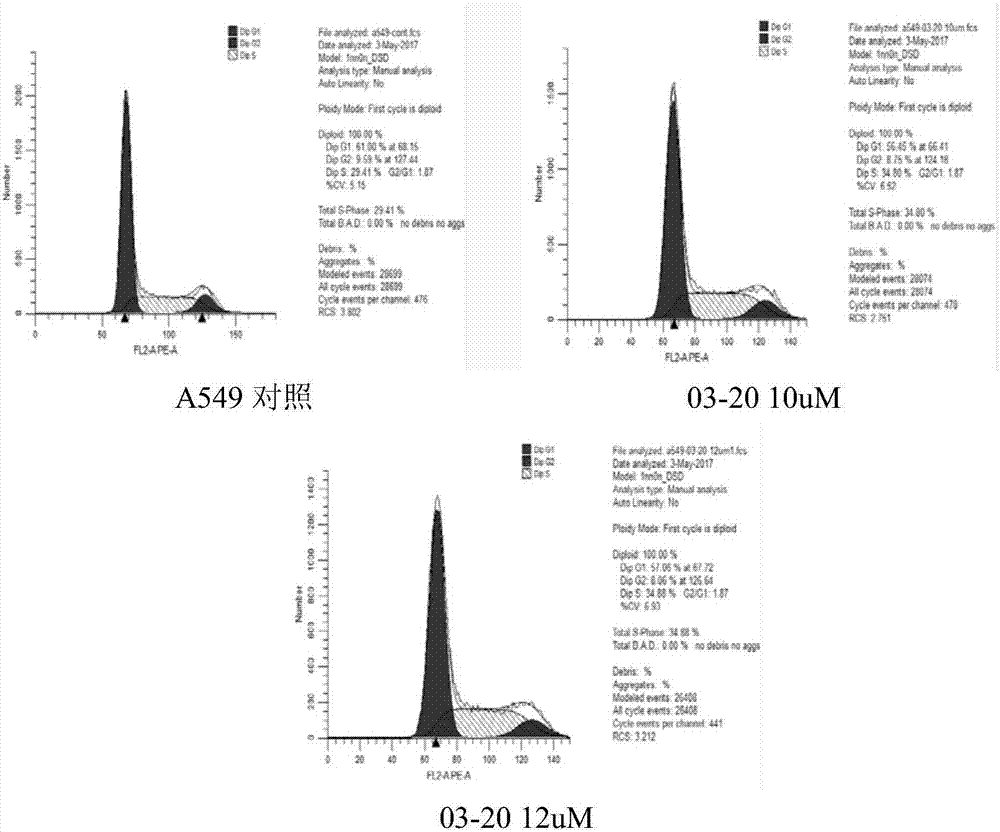
Alkoxyl terminal group oligo-PEG modified aminopyrimidine derivative and antitumor application
An alkyl compound technology, applied in the field of pyrazolo[3,4-d]pyrimidine derivatives, can solve the problems of low bioavailability, high toxicity and side effects, and low specificity, so as to promote apoptosis, Promising effect of inhibiting cell proliferation and clinical medicinal use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation of embodiment 1 compound of the present invention
[0043]
[0044] Compound 1 (269mg, 1mmol) was dissolved in 1,4-dioxane / water (4:1) 150ml solution, and transferred to a 250ml round bottom flask, and then added (1.38g, 10mol) to the reaction solution Potassium carbonate, after stirring at room temperature for three minutes, add the catalyst bis-diphenylphosphinoferrocene palladium chloride (73.1 mg, 0.1 mol), heat the reaction mixture to 100 ° C, detect the reaction by TLC, after the reaction is completed , first, after the reaction solution is cooled, add 50ml of distilled water, then transfer to a separatory funnel, add 100ml of dichloromethane to extract 3 times, combine the organic phases, add anhydrous sodium sulfate, spin dry the organic solvent, and separate through a silica gel column Intermediate compound 2 (200 mg, 70%) was obtained, (dichloromethane:(dichloromethane:methanol)=100:2.5).
[0045] 1 H NMR (600MHz, DMSO-6d) δ9.83(s, 1H, HOA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com