Polypeptide and application thereof to preparation of medicine for treating and preventing tumors
A drug and species-specific technology, applied in anti-tumor drugs, drug combinations, peptides, etc., can solve the problems of cell membrane protease hydrolysis, impermeability of linear polypeptides, weak binding ability of polypeptides and acting proteins, etc. Significant, safe to use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The synthesis of embodiment 1 polypeptide
[0039] For the amino acid sequence of polypeptide A2, see SEQ ID No.8 in the sequence listing. Polypeptide A2 was synthesized and purified by Beijing Saibaisheng Gene Technology Co., Ltd.
[0040] Two unnatural amino acids S-pentylalanine (S5) were introduced for solid-phase polypeptide chain synthesis. After the solid-phase polypeptide chain is synthesized, ruthenium is used as a catalyst to perform olefin metathesis reaction (RCM) cyclization to obtain the target polypeptide. Finally, the target polypeptide is cleaved from the resin for purification. The steps of solid-phase polypeptide chain synthesis and purification were completed by China Peptide Biochemical Co., Ltd. Among them, two S-pentylalanines are inserted into the i, i+3 or i, i+4 positions in the amino acid sequence of the polypeptide A2, thereby obtaining modified polypeptides of different sequences (see the sequence listing for the amino acid sequence SEQ ...
Embodiment 2
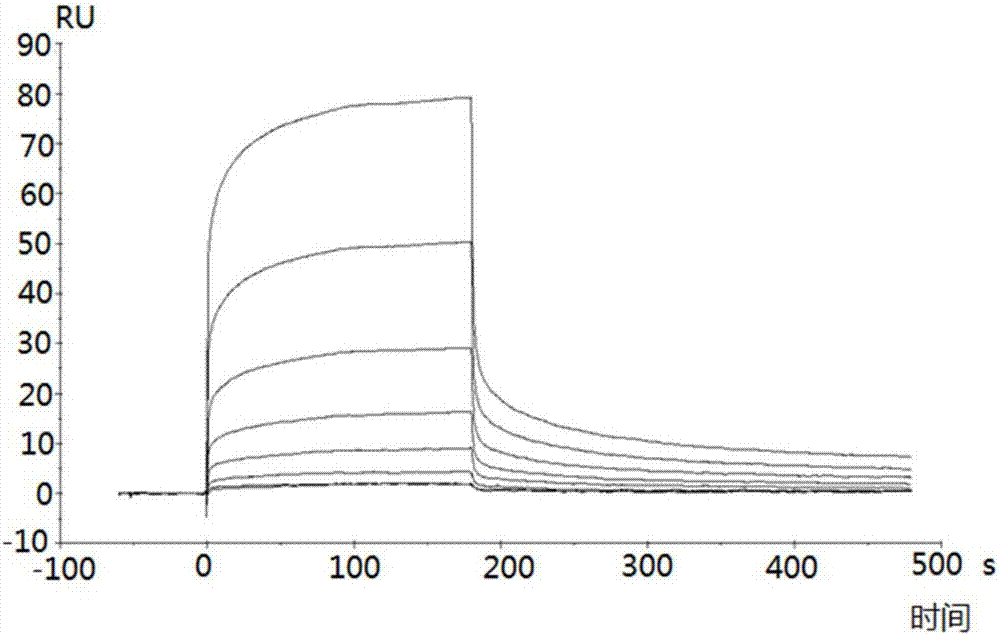
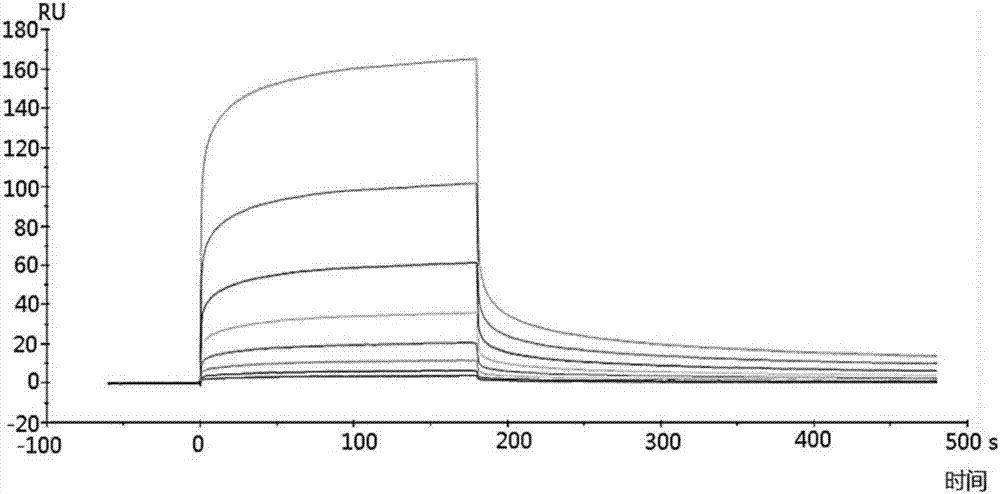
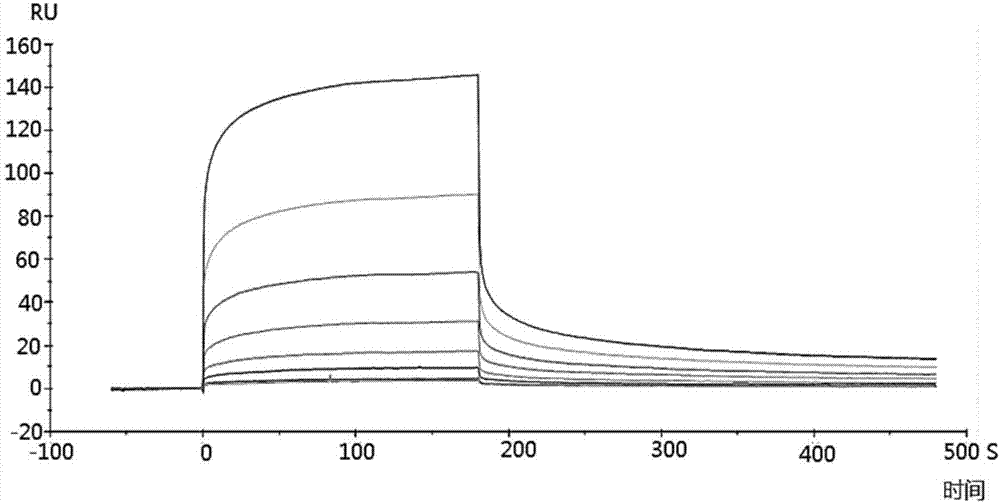
[0048] Example 2 Detection of binding ability of polypeptide and TRB3 protein by surface plasmon resonance
[0049] The surface plasmon resonance experiment was carried out in a surface plasmon resonance instrument Biacore T200, and the operation steps were carried out according to the instructions of the plasmon resonance instrument Biacore T200. Specific steps are as follows:
[0050] 1. The purified TRB3 protein (purchased from RD Company) was amino-coupled to a CM5 chip (purchased from GE Company), and the unbound protein was eluted at a flow rate of 10 μL / min, and the surface of the chip was equilibrated for 2 hours. For the specific steps of amino coupling, elution and equilibration, please refer to the relevant instructions of GE's CM5 chip.
[0051] 2. Automatically inject 250 μL of the S1-S7 and A2 polypeptide fragments prepared in Example 1 at different concentrations (800, 400, 200, 50, 12.5, 6.25 and 3.125 nM), and conduct the entire surface plasmon resonance expe...
Embodiment 3
[0055] Embodiment 3 Circular dichroism method detects the alpha helical rate of polypeptide
[0056] The α-helix rate of the polypeptide was detected with a circular dichroism spectrometer (purchased from Jasco, Japan). The polypeptides A2, S1, S2, S3, S4, S5, S6 and S7 prepared in Example 1 were dissolved in PBS solution, and the concentration of the circular dichroism spectrometer was adjusted to 1 mg / mL. The results are shown in Table 2 . Table 2 shows that the α-helix rate of polypeptides S1, S2, S3, S4, S5, S6 and S7 is significantly higher than that of polypeptide A2, because the α-helix secondary structure of polypeptides mediates the binding of polypeptides to TRB3 protein, therefore, polypeptides S1-S7 The increase of the α-helix rate of the protein is related to the increase of its ability to bind to TRB3 protein, as well as the proliferation and metastasis of various tumor cells. Among them, the α-helix rate refers to the percentage of the number of peptides that ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com