Organic electroluminescent material taking xanthene as core and application of organic electroluminescent material
A luminescent material, electroluminescence technology, applied in luminescent materials, organic chemistry, circuits, etc., can solve the problems of disparity, and achieve the effect of significantly improving lifespan, improving thermal stability, and balance of electron and hole distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
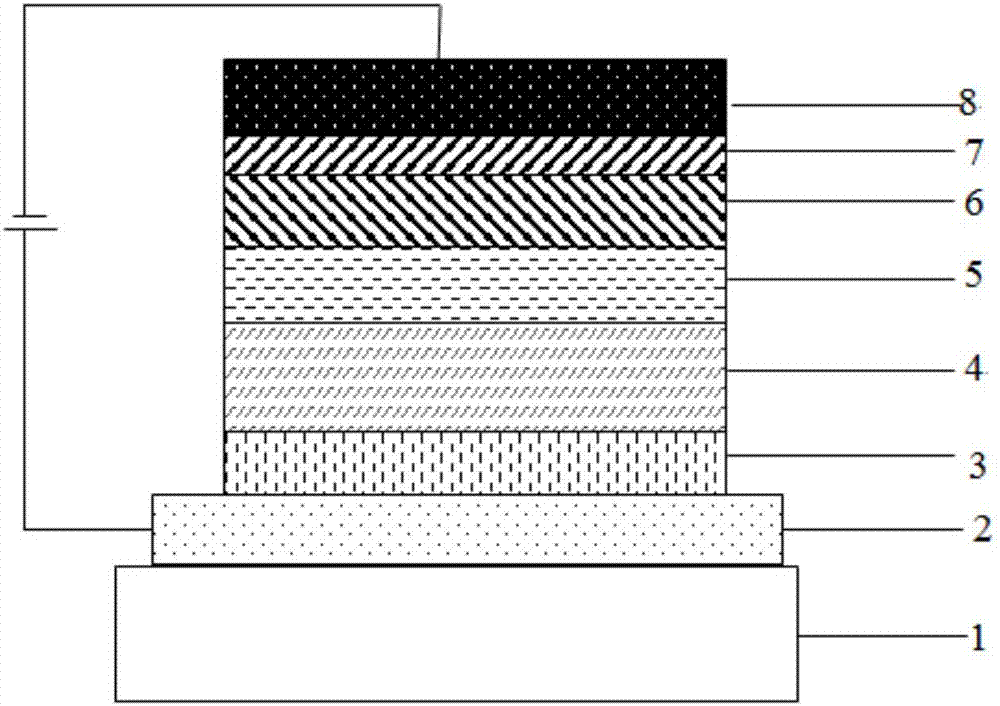
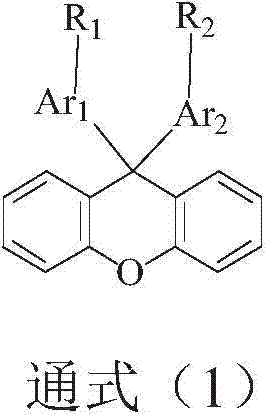
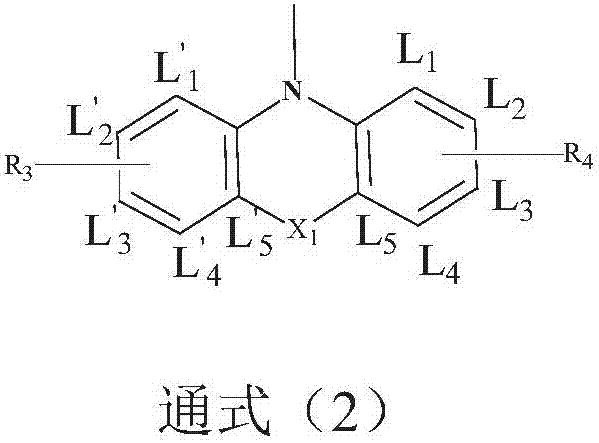
Image
Examples
Embodiment 1
[0048] The synthesis of embodiment 1 compound 1:
[0049] synthetic route:
[0050]
[0051] In a 250ml four-neck flask, under a nitrogen atmosphere, add 11.8g of p-1,4-dibromobenzene (0.05mol) and 1.44g of Mg powder (0.06mol), 60ml of tetrahydrofuran, heat and reflux for 4 hours, the reaction is complete, and formatting reagents;
[0052] Dissolve 9.8g xanthanone (0.05mol) in 50ml tetrahydrofuran, add the above-mentioned Grignard reagent dropwise, react at 60°C for 24 hours, a large amount of white precipitate is formed, and finally add saturated NHCl 4 Grignard salt was converted into alcohol; after the reaction was completed, extracted with ether, dried and rotary evaporated, and purified on a silica gel column with a mixed solvent of petroleum ether:dichloromethane (3:2) to obtain slightly yellow compound C (90% yield); Using DEI-MS to identify the compound, formula C 19 h 13 BrO, detection value [M+1] + = 353.85, calculated value 353.21;
[0053] Dissolve 14.1g o...
Embodiment 2
[0056] The synthesis of embodiment 2 compound 3:
[0057] synthetic route:
[0058]
[0059]
[0060] Dissolve 14.1g of compound C (0.04mol) and 16.3g of iodobenzene (0.08mol) in 1:2 equivalents in 100ml of dichloromethane, add 10ml of boron trifluoride etherate complex dropwise at room temperature, and react for 30 Minutes, add 20ml ethanol and 20ml water to quench the reaction, extract with dichloromethane (20ml*3), dry and rotary evaporate, petroleum ether silica gel column purification, use ethanol: dichloromethane recrystallization to obtain compound E (yield is 82% ); use DEI-MS to identify the compound, molecular formula C 25 h 16 BrIO, detection value [M+1] + =538.82, calculated value 537.94;
[0061] In a 250ml three-necked flask, under the protection of nitrogen, add 10.8g (0.02mol) compound E, 6.3g (0.03mol) 9,9-dimethyl-9,10-dihydroacridine, 5.5g (0.04mol) ) Potassium carbonate, 0.4g cuprous iodide, 200ml dioxane, heated to reflux for 24 hours, took a sa...
Embodiment 3
[0064] The synthesis of embodiment 3 compound 8:
[0065]
[0066] Prepared according to the synthetic method of compound 1 in Example 1, the difference is that compound G is used to replace 10H-phenoxazine;
[0067] Using DEI-MS to identify the compound, formula C 49 h 32 N 2 o 2 , detection value [M+1] + =681.23, calculated value 680.25.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com