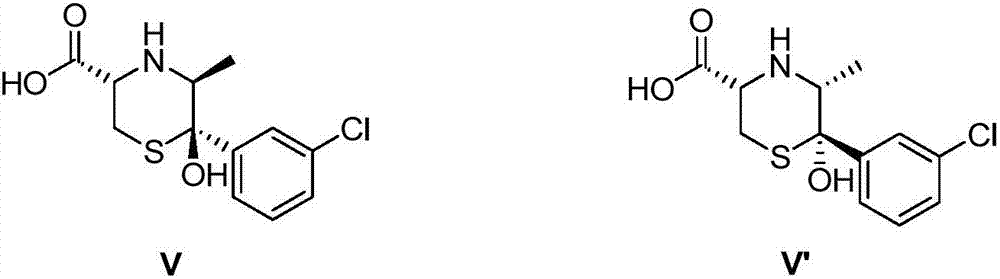Method for synthesizing impurity isomers of bupropion hydrochloride sustained-release tablets and application of impurity isomers
A technology of bupropion hydrochloride and its synthetic method, which is applied in the field of chemical pharmacy, can solve problems such as unfavorable quality control of bupropion hydrochloride, achieve chiral control stereospecificity, simple and efficient synthetic route, and improve accurate positioning and qualitative Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055](1) Preparation of methyl (S)-2-amino-3-(tritylmercapto)propionate: Add 80mL N,N-dimethylformamide and 20g D-cysteine to a 250mL single-necked bottle Methyl ester hydrochloride (compound I, 0.12 mol) was stirred at room temperature to dissolve, and 45.2 g of triphenylchloromethane (0.16 mol) was added, and reacted at 20-25°C for 48 hours. Complete conversion of starting material was monitored by TLC. Add the reaction solution to 180mL of 10% sodium acetate solution, stir to obtain a white viscous oil, remove the upper water layer, dissolve the white viscous oil with 200mL of dichloromethane, add 40mL of saturated sodium chloride solution to wash, divide Remove the aqueous phase. The organic phase was concentrated under reduced pressure to obtain 42.7 g of a yellow oil, with a yield of 97.1%.
[0056] (2) Preparation of (S)-2-(1-(3-chlorophenyl)-1-oxopropane-2-amino)-3-(tritylmercapto)propionic acid methyl ester: 100mL one-mouth bottle Add 16mL N,N-dimethylformamide,...
Embodiment 2
[0065] (1) Preparation of methyl (S)-2-amino-3-(benzyloxycarbonylmercapto)propionate: 17.6g of D-cysteine methyl ester hydrochloride (compound I, 0.10mol) was added to a 500mL three-neck flask , 200mL 1N NaHCO 3 Solution, stir to dissolve, then add 100mL methyl tert-butyl ether, cool down to 0°C, add 17.3g benzyl chloroformate (0.13mol) at one time, stir at 0°C for 1h, slowly heat up to 10°C, at this temperature Stirring was continued for 1 h, filtered with suction, the filter cake was washed with water, then with acetone and methyl tert-butyl ether, and dried in vacuo to obtain 20.7 g of a white solid with a yield of 75%.
[0066] (2) Preparation of (S)-2-(1-(3-chlorophenyl)-1-oxopropane-2-amino)-3-(benzyloxycarbonylmercapto)propionic acid methyl ester: in a 100mL single-necked bottle Add 16mL N,N-dimethylformamide, 10g (R)-2-amino-3-(benzyloxycarbonylmercapto) propionate methyl ester (37mmol), stir at room temperature to dissolve, add 6.7g triethylamine, and then Add 12....
Embodiment 3
[0071](1) Preparation of (S)-2-amino-3-(benzylmercapto)propionic acid methyl ester: add 50mL dichloromethane, 20mL water, 10g D-cysteine methyl ester hydrochloride to a 250mL single-necked bottle (Compound I, 58mmol), 9.6g of potassium carbonate, cooled in an ice bath, slowly added 12.2g of benzyl bromide (71mmol), removed from the ice bath, stirred overnight at 35-40°C, separated the layers, and extracted the aqueous phase with dichloromethane for 2 Once, the organic phase was washed by adding 20 mL of saturated sodium chloride solution, the water phase was separated, and the organic phase was concentrated under reduced pressure to obtain 11.2 g of yellow oil, with a yield of 85.3%.
[0072] (2) Preparation of (S)-2-(1-(3-chlorophenyl)-1-oxopropane-2-amino)-3-(benzylmercapto)propionic acid methyl ester: add 16mL N,N-dimethylformamide, 10g (R)-2-amino-3-(benzylmercapto) propionate methyl ester (44mmol), stirred at room temperature to dissolve, add 7.8g potassium carbonate, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com