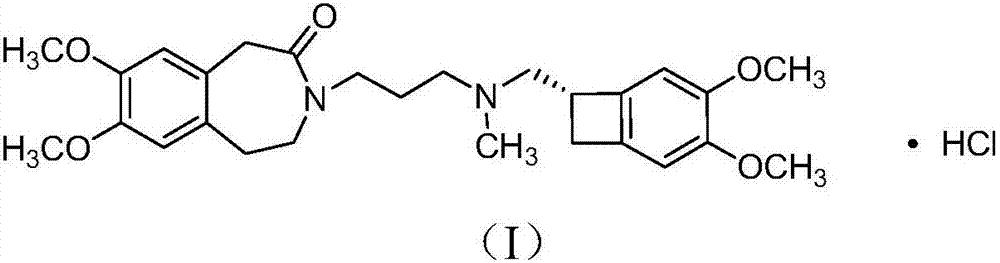
Method for preparing ivabradine hydrochloride alpha crystal form
A technology of ivabradine hydrochloride and its crystal form, which is applied in the fields of organic chemistry and organic chemistry, and can solve problems such as difficult removal of residual solvents and low product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
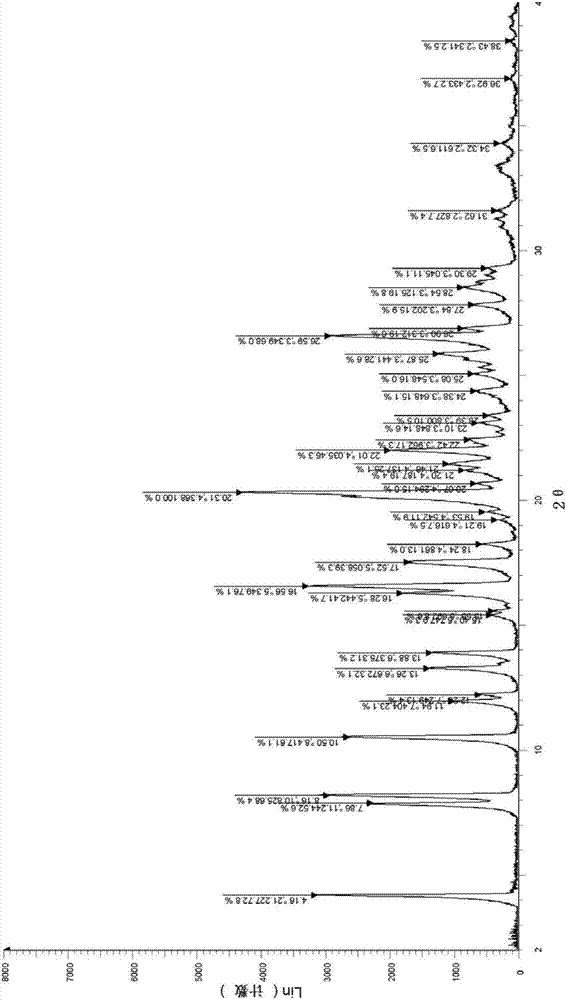
[0044]In 100mL (10 times, V / W) toluene, add 10.0g ivabradine hydrochloride (δ crystal form) obtained according to the method described in the patent specification EP0534859, heat up to reflux, stir for 0.5h, cool to room temperature, reduce Pressure filtration, and then drying at 60° C. under vacuum for 6 h, yielded 9.7 g of white solid powder, with a yield of 97.0%.
[0045] Analyzing assay results
[0046]
Embodiment 2
[0048] In 100mL (10 times, V / W) toluene, add 10.0g ivabradine hydrochloride (δd crystal form) obtained according to the method described in the patent specification EP0534859, heat up to reflux, stir for 0.5h, cool to room temperature, reduce Press filter, and then dry at 60°C under vacuum for 6 hours to obtain 9.8 g of white solid powder with a yield of 98.0%.
[0049] Analyzing assay results
[0050]
Embodiment 3
[0052] In 100mL (10 times, V / W) toluene, add 10.0g ivabradine hydrochloride (beta crystal form) obtained according to the method described in the patent specification CN1827600B, heat up to reflux, stir for 1.5h, cool to room temperature, reduce Press filter, and then dry at 60°C under vacuum for 6 hours to obtain 9.8 g of white solid powder with a yield of 98.0%.
[0053] Analyzing assay results
[0054]
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com