Preparation method and application of ivabradine structural analogue or acid salt thereof
An ivabradine technology with a similar structure, applied in the field of medicine, can solve the problems of complicated operation, long steps, difficult side chain synthesis, etc., and achieve the effects of high yield, simple operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] The preparation of embodiment 1 formula (I-3) compound
[0056] Add 150ml of N-methylpyrrolidone into a 250ml reaction flask, add 15.0g of ivabradine (ie, the compound of formula (II-1)) and 10.0g of manganese dioxide under stirring, and stir and heat up to 90-100°C for complete reaction. The reaction solution was lowered to room temperature, filtered to obtain a filtrate, 50ml of saturated brine and 100ml of dichloromethane were added to the filtrate, the organic phase was extracted and separated, dried over anhydrous sodium sulfate, and the organic phase was concentrated under reduced pressure to obtain a brown oily concentrate. The concentrate was dissolved in 30ml of tetrahydrofuran, and an appropriate amount of concentrated hydrochloric acid was added dropwise under stirring to form a salt, and an appropriate amount of n-heptane was added to slowly crystallize to obtain a brown solid compound of formula (I-3), with a yield of 58%.
[0057]
Embodiment 2
[0058] The preparation of embodiment 2 formula (I-3) compound
[0059] Add 100ml of acetonitrile to a 250ml reaction flask, add 20.0g of ivabradine, 12.0g of tert-butyl hydroperoxide and 1.2g of ketone iodide while stirring, and heat up to reflux with stirring. The reaction solution was cooled to 40-50°C, concentrated under reduced pressure to remove the organic solvent, then added 50ml of water and 50ml of ethyl acetate, extracted and separated to obtain an organic phase, dried over anhydrous sodium sulfate, added 20ml of methanol, and added dropwise an appropriate amount of Concentrated hydrochloric acid was used to form a salt, and the organic phase was concentrated under reduced pressure to obtain a brown foamy compound, which was beaten with methyl tert-butyl ether to obtain a brown solid compound of formula (I-3), with a yield of 62%.
Embodiment 3
[0060] The preparation of embodiment 3 formula (I-3) compound
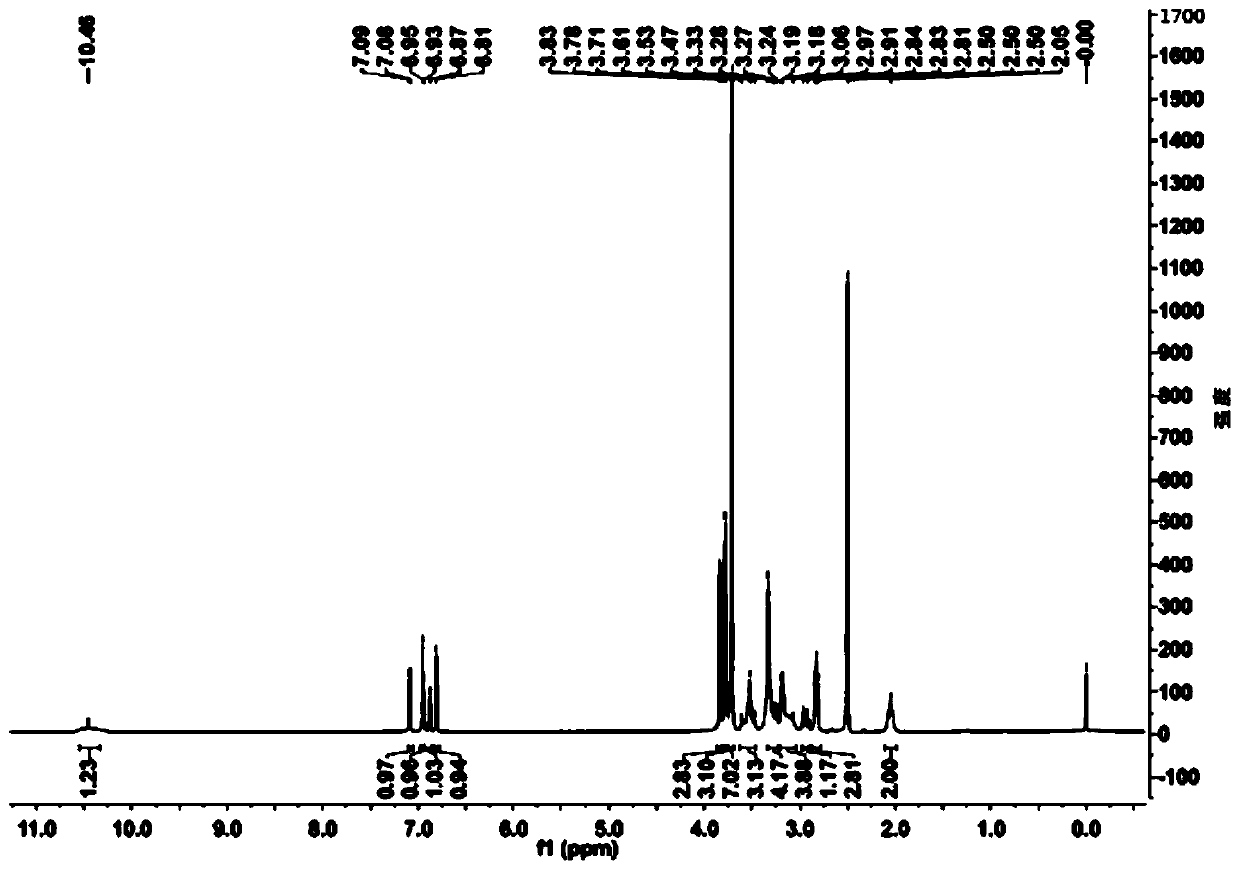
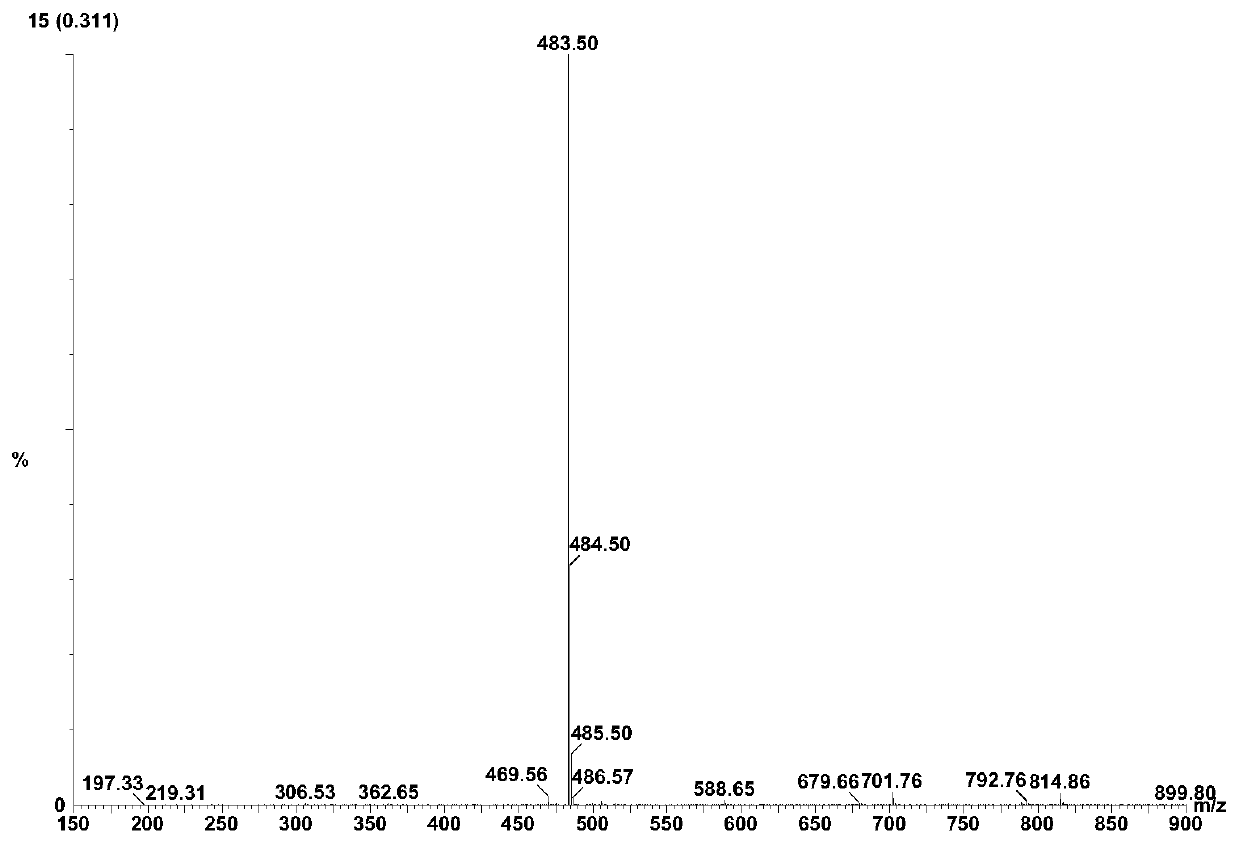
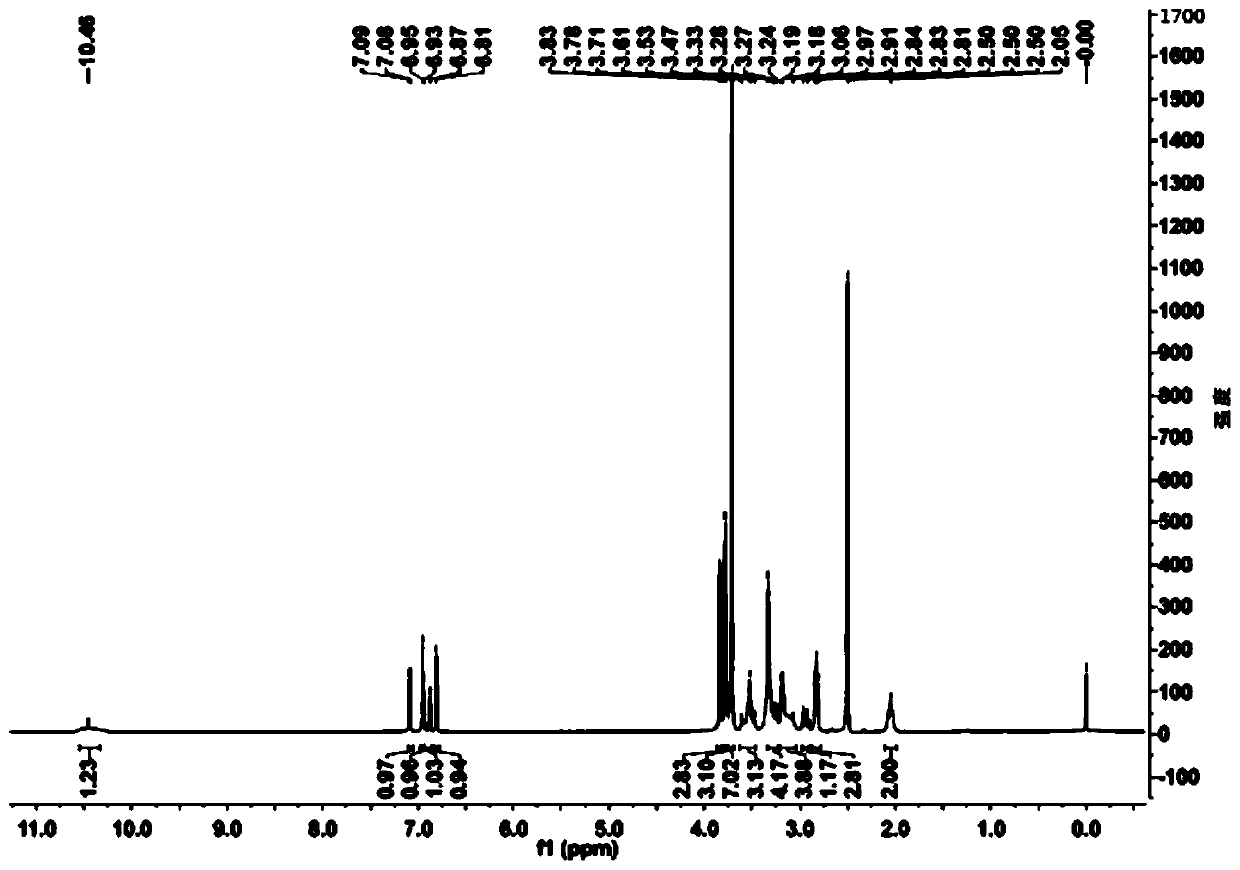
[0061] Add 20ml of water and 80ml of 1,4-dioxane into a 250ml reaction bottle, add 18.0g of ivabradine and 9.0g of selenium dioxide under stirring, and stir and heat up until the reflux reaction is complete. The reaction solution was cooled to 50-60°C, concentrated under reduced pressure to remove the organic solvent, then added 50ml of water and 100ml of dichloromethane, extracted and separated to obtain an organic phase, dried over anhydrous sodium sulfate, concentrated under reduced pressure to obtain a brown oily concentrate . The concentrate was dissolved in 20ml of acetone, and an appropriate amount of concentrated hydrochloric acid was added dropwise with stirring to form a salt, and an appropriate amount of n-hexane was added to slowly crystallize to obtain a brown solid compound of formula (I-3), with a yield of 81%. HPLC purity test 99.1%; 1 H-NMR (400MHz, d 6 -DMSO)δ: 10.46(s,1H), 7.08(d,1H), 6.95(s,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com