Hydrogen-resistance permeation layer on surface of metal hydride and preparation method thereof
A hydride and permeable layer technology, applied in metal material coating process, coating, solid-state diffusion coating, etc., can solve the problem of weak bonding force between the coating and the hydride matrix, difficult to implement hydrogen barrier film, and the film layer is not dense. Complete and other problems, to achieve the effect of excellent hydrogen barrier performance, moderate thickness and dense film layer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1. Degrease, clean and polish the surface of Φ30mm×30mm zirconium hydride, and the H / Zr of zirconium hydride is 1.65.
[0034] 2. Loading and heat-resistant glass packaging
[0035] ① Weigh 1.5g urea with a balance;
[0036] ② Put urea and zirconium hydride in a high-temperature-resistant glass tube, vacuum seal, and the pressure inside the high-temperature-resistant glass tube is 1×10 -5 Pa.
[0037] 3. Preparation of hydrogen-resistant permeable layer containing carbon, nitrogen and oxygen
[0038] Put the encapsulated glass tube in a temperature-controlled tube furnace, raise it from room temperature to 500°C at a rate of 300°C / h, keep it at 500°C for 100 hours, then cool it down to 300°C at a rate of 600°C / h, and then cool it naturally to room temperature to obtain a hydrogen permeable barrier layer containing carbon, nitrogen and oxygen.
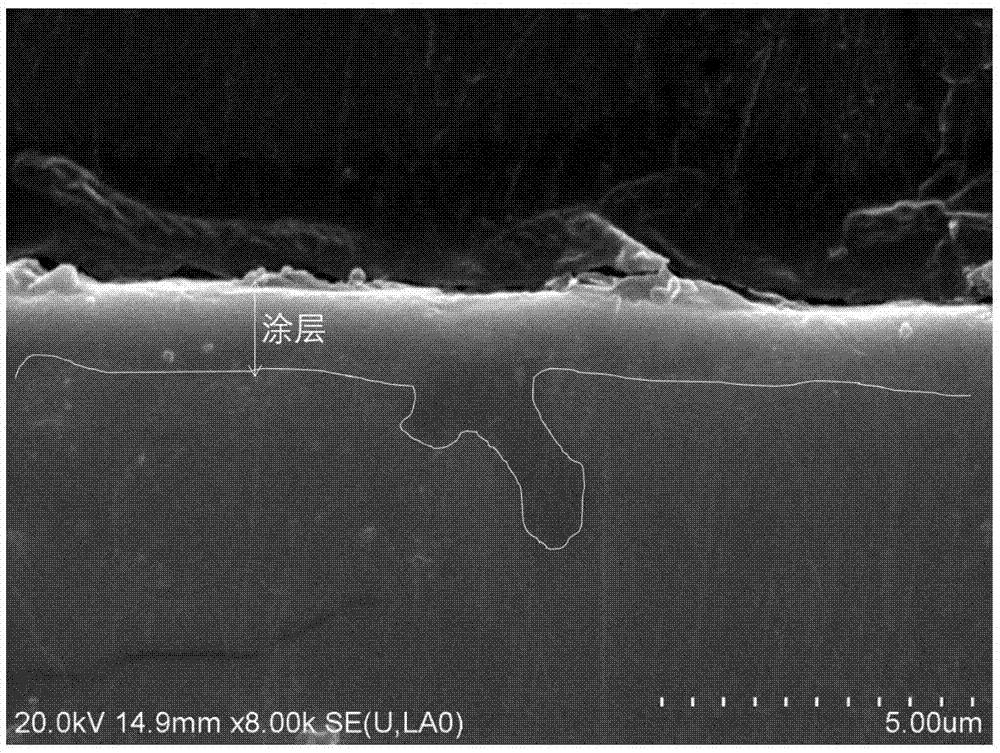
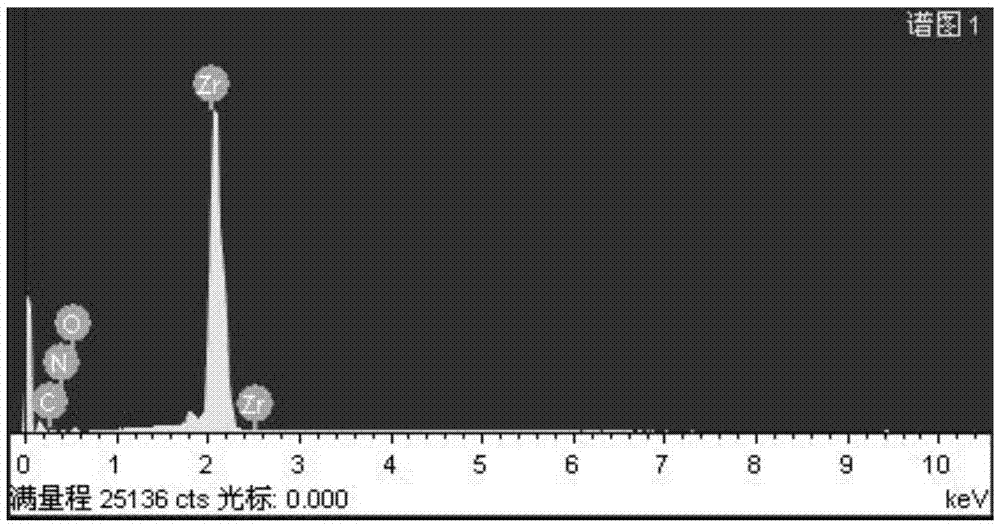
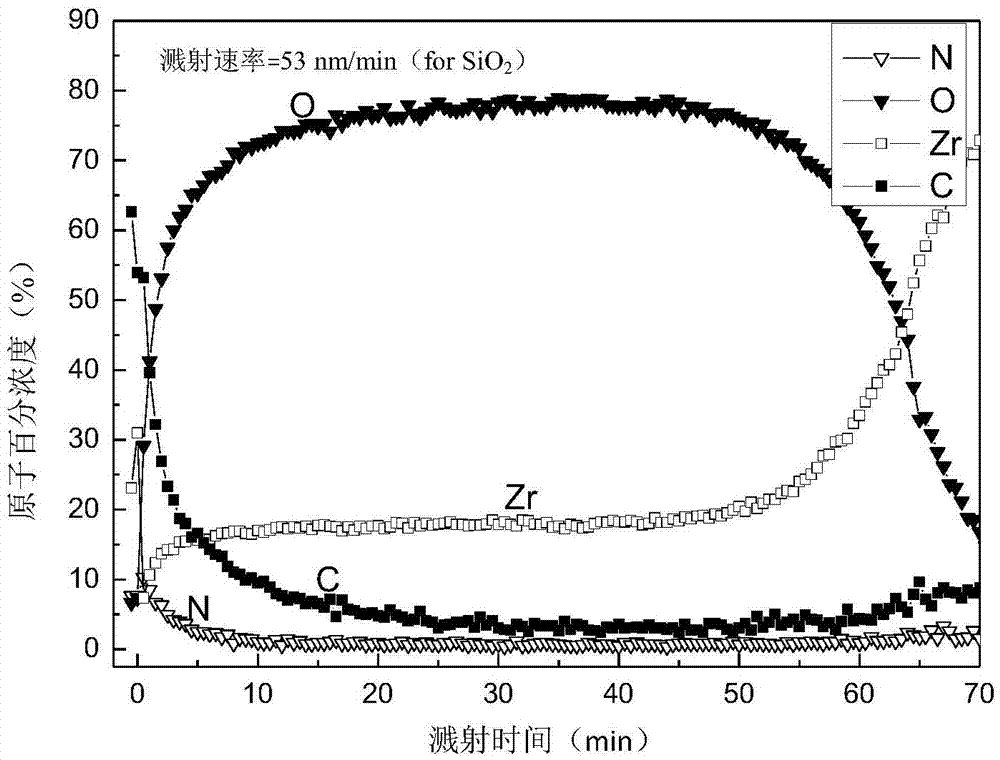
[0039] The surface AES analysis of the hydrogen barrier permeation layer adopts the PHI-700 nanometer scanning Auger system...
Embodiment 2
[0042] 1. Degrease, clean and polish the surface of Φ30mm×30mm zirconium hydride, the H / Zr of zirconium hydride=1.85.
[0043] 2. Loading and quartz tube packaging
[0044] ① Use a balance to weigh 5g of acrylamide;
[0045] ②Place acrylamide and zirconium hydride in a quartz tube for vacuum packaging, the pressure inside the quartz tube is 1×10 -3 Pa.
[0046] 3. Preparation of hydrogen-resistant permeable layer containing carbon, nitrogen and oxygen
[0047] Put the packaged quartz tube in a temperature-controlled tube furnace, raise it from room temperature to 400°C at a rate of 600°C / h, keep it at 400°C for 200h, and then raise it from room temperature to 500°C at a rate of 300°C / h , kept at 500° C. for 100 h, and finally cooled naturally in the furnace to room temperature to obtain a hydrogen-resistant permeable layer containing carbon, nitrogen and oxygen, and its hydrogen permeability level is equivalent to that of the carbon, nitrogen and oxygen-containing coating i...
Embodiment 3
[0049] 1. Degrease, clean and polish the surface of Φ20mm×20mm zirconium hydride, and the H / Zr of zirconium hydride is 1.9.
[0050] 2. Loading and stainless steel pipe welding and sealing
[0051] ① Take 5g of ammonium carbonate with a balance;
[0052] ②Put the ammonium carbonate and zirconium hydride in the stainless steel tube, argon arc welding, the pressure inside the stainless steel tube is 101kPa.
[0053] 3. Preparation of hydrogen-resistant permeable layer containing carbon, nitrogen and oxygen
[0054] The stainless steel tube is placed in a temperature-controlled tube furnace, raised from room temperature to 200°C at a rate of 300°C / h, and kept at 200°C for 1 hour; then raised to 500°C at a rate of 100°C / h, and kept at 500°C for 50 hours ; After that, the temperature was raised to 600°C at a rate of 10°C / h, and kept at 600°C for 200h; then the temperature was lowered to 300°C at a rate of 600°C / h, and then naturally cooled to room temperature to obtain a hydrogen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com