Application of bifidobacterium infantis in foods or medicines for preventing and treating food allergy
A bifidobacterium, food allergy technology, applied in the direction of food science, drug combination, allergic diseases, etc., can solve problems such as food allergy that cannot be completely cured, and achieve the effect of preventing food allergy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example
[0015] Experimental animals: BALB / c mice, male, 6 weeks old, weighing 18-22g, mouse feed, bedding, mouse cages and other items are provided by the Experimental Animal Center of the Fourth Military Medical University. All animal experiments in this experiment are strictly in accordance with " The Experimental Animal Regulations of the Fourth Military Medical University was implemented, and the animal feeding was completed in the biosafety laboratory (BSL-II): BALB / c mice were raised according to SPF level and kept in special validity cages, and they drank sterilized deionized water freely. The temperature of the animal room is 19-25°C, the humidity is 55-65%, the light is 10-12 hours / day, and the mouse cage is cleaned and disinfected twice a week.
[0016] Experimental strain: Bifidobacterium infantis CGMCC NO.0313-2 strain (Bifidobacterium infantisCGMCC NO.0313-2), identified by the Institute of Microbiology, Chinese Academy of Sciences, provided by Shandong Kexing Biological P...
experiment example 1
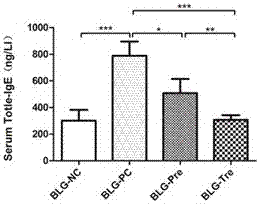
[0033] After 1 week of adaptive feeding, the labels were sorted according to body weight, and 4 mice with adjacent body weights were used as a block, and random numbers were generated, and grouped according to random numbers, 40 BALB / c male mice were randomly divided into four groups, respectively They were: normal control group (BLG-NC), positive control group (BLG-PC), prevention group (BLG-Pre), treatment group (BLG-Tre), 10 rats in each group. Modeling was started after the grouping was completed, and Bifidobacterium infantum was intervened. (1) Modeling: the normal control group was modeled with normal saline as a control, and the other three groups were sensitized on the 7th, 14th and 21st day, and the sensitization solution was administered intragastrically 2ml / time, and 2ml of the sensitization solution contained 20mg ß- Lactoglobulin (BLG, Sigma, Buchs, Switzerland) + adjuvant 10 µg cholera toxin (CTX, List Biological Laboratories, Campbell, CA, USA); on the 28th day ...
experiment example 2
[0035] (1) Preparation of BLG sensitization solution: frozen at -20°C, ready for use, weigh 1g of BLG and 0.5mg of CTX powder with a balance, mix them evenly, dissolve them in 10ml of normal saline, centrifuge at 750rmp for 15 minutes, and discard A small amount of supernatant, adjust the suspension to 10ml, and ensure that the final concentration of BLG is 0.1g / ml; (2) Preparation of BLG excitation solution: prepare it now, weigh 1g of BLG with a balance, and dissolve it in 2ml of normal saline After mixing, the suspension was about 3ml, and each mouse was gavaged with 0.3ml of the suspension when challenged.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com