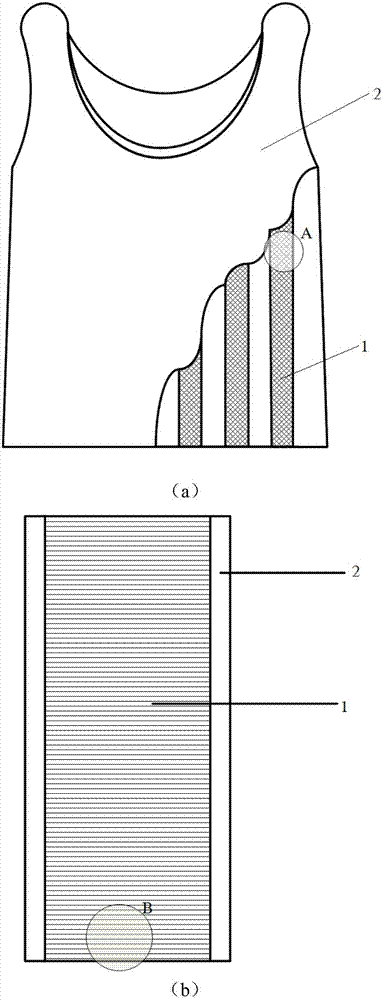
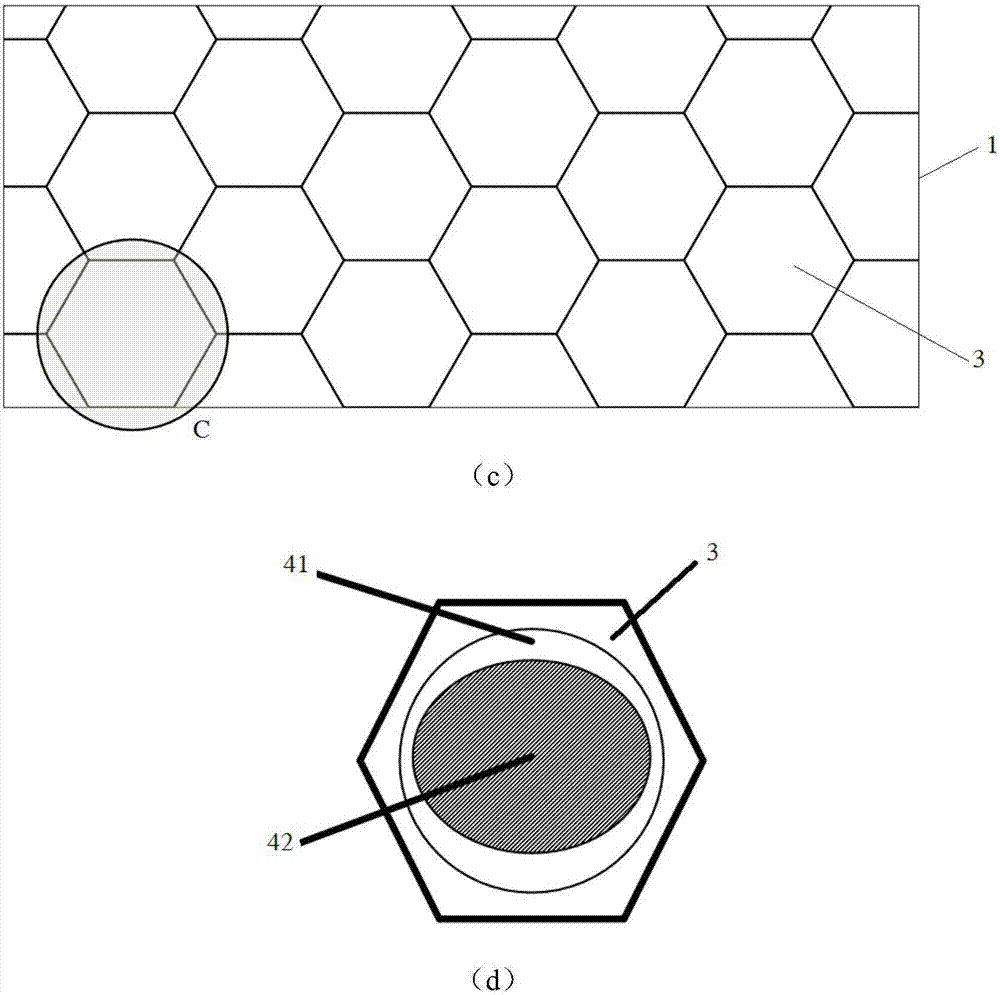
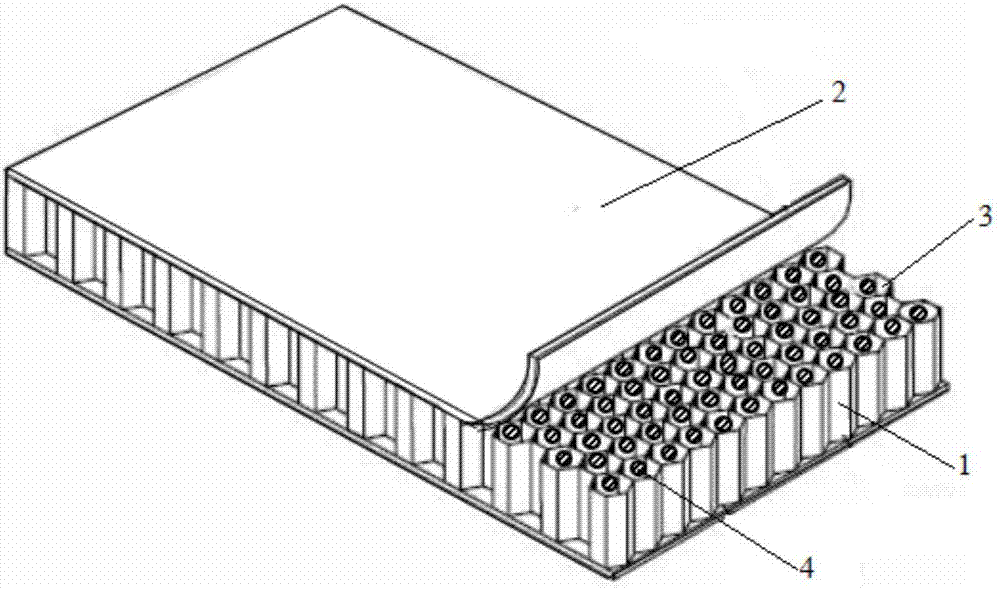
Wearable equipment based on composite phase change material and production method of wearable equipment
A technology of composite phase change materials and equipment, applied in the direction of heat exchange materials, chemical instruments and methods, clothing, etc., can solve the problems of poor encapsulation and easy leakage of thermal conductivity of organic phase change materials, and achieve non-corrosive chemical properties, The effect of shielding heat radiation and preventing deformation and leakage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Preparation of composite phase change materials
[0039] Take lauric acid and stearic acid with a mass ratio of 60.3:39.7, add them to a beaker with a total mass of 50 g, put them on a magnetic stirrer with heating function, set the heating temperature to 100 ° C, and the rotation speed to 1800 r / min. Until it is completely melted, the eutectic mixture of lauric acid and stearic acid is obtained, and then cooled to room temperature to obtain a composite phase change material.
[0040] (2) Measuring the physical parameters of composite phase change materials
[0041] Using differential scanning calorimeter, protective atmosphere: nitrogen; flow rate: 15ml / min; heating rate: 5K / min, test temperature range 20℃~50℃. Draw the DSC curve of the composite phase change material of lauric acid and stearic acid, and analyze the phase change temperature and latent heat of phase change. The obtained results are t 1 = 34.316°C and H 1 = 189.73 kJ / kg.
[0042] (3) preparation ...
Embodiment 2
[0050] (1) Synthesis of composite phase change materials
[0051] Take lauric acid and myristic acid with a mass ratio of 58:42, add them to a beaker with a total mass of 50 g, put them on a magnetic stirrer with heating function, set the heating temperature to 100 ° C, and the rotating speed to 1400 r / min until Completely melted to obtain the eutectic mixture of lauric acid and myristic acid, and then cooled to room temperature to obtain a composite phase change material.
[0052] (2) Measuring the physical parameters of composite phase change materials
[0053] Using differential scanning calorimeter, protective atmosphere: nitrogen; flow rate: 15ml / min; heating rate: 5K / min, test temperature range 20℃~50℃. Draw the DSC curve of the composite phase change material of lauric acid and myristic acid, and analyze the phase change temperature and latent heat of phase change. The obtained results are t 2 =34.273°C, H 2 = 162.801 kJ / kg.
[0054] (3) preparation of microcapsules...
Embodiment 3
[0062] (1) Synthesis of composite phase change materials
[0063] Take lauric acid and palmitic acid with a mass ratio of 69:31, add them to a beaker with a total mass of 50 g, put them on a magnetic stirrer with a heating function, set the heating temperature to 100 °C, and the rotation speed to 1200 r / min until complete melting to obtain a eutectic mixture of lauric acid and palmitic acid, and then cooling to room temperature to obtain a composite phase change material.
[0064] (2) Measuring the physical parameters of composite phase change materials
[0065] Using differential scanning calorimeter, protective atmosphere: nitrogen; flow rate: 15ml / min; heating rate: 5K / min, test temperature range 20℃~50℃. Draw the DSC curve of the composite phase change material of lauric acid and stearic acid, and analyze the phase change temperature and latent heat of phase change. The obtained results are t 3 =33.283°C, H 3 = 166.54 kJ / k.
[0066] (3) preparation of microcapsules
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| phase transition temperature | aaaaa | aaaaa |
| UV shielding rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com