A method for preparing nanoscale lithium manganese phosphate
A lithium manganese phosphate, nano-scale technology, applied in nanotechnology, nanotechnology, chemical instruments and methods, etc., can solve the problems of high energy consumption, uneven product particle size, and easy agglomeration of solid-phase methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
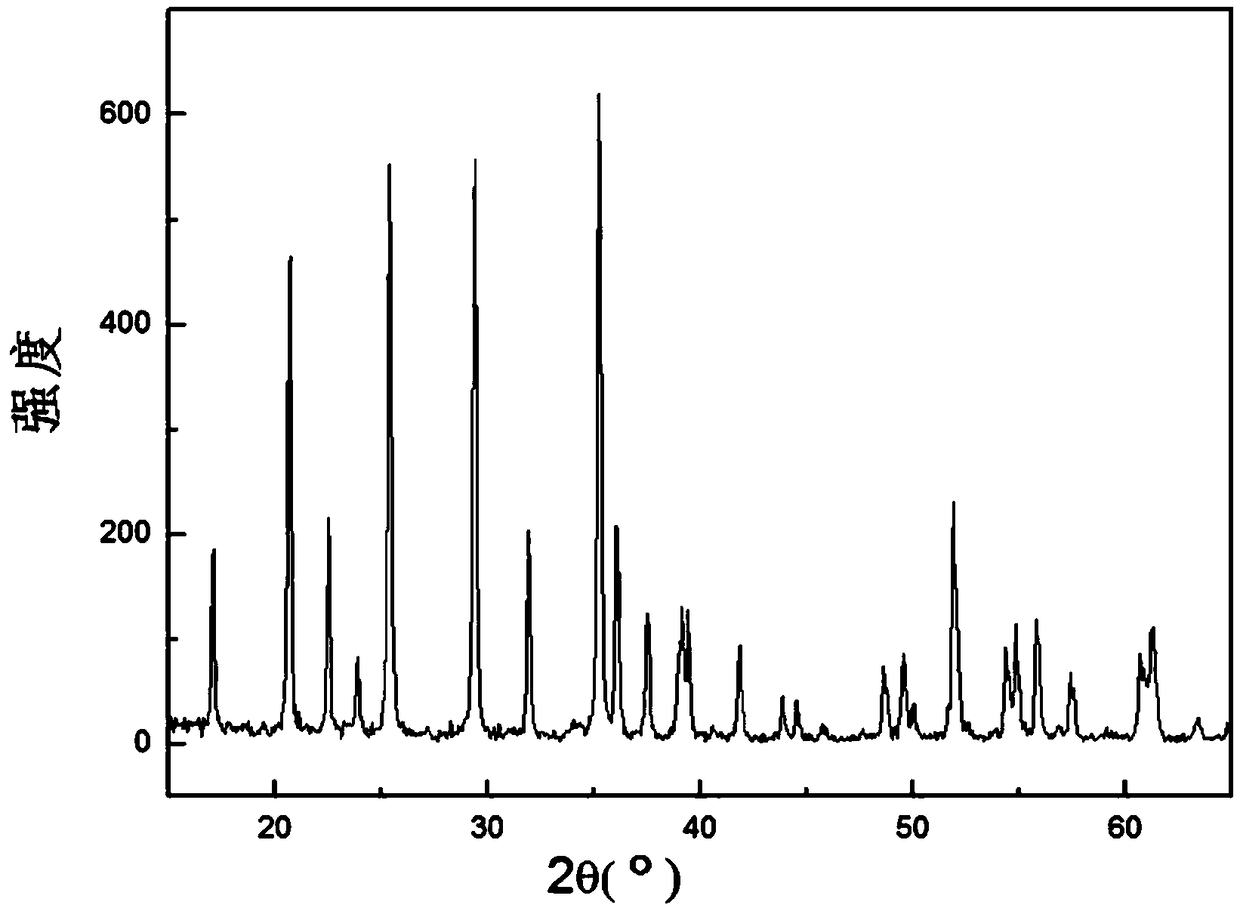
[0034] refer to figure 1 , In this embodiment, a method for preparing nanoscale lithium manganese phosphate is provided. The method comprises the steps of:
[0035] S1, preparing aluminum grid-based lithium phosphate pole pieces;
[0036] S1, using the manganese sheet as the anode, electrolyzing in the imidazole ionic liquid, introducing manganese ions into the imidazole ionic liquid to form the imidazole ionic liquid containing manganese ions;
[0037] S3. Electrodeposition is performed in an imidazole ionic liquid containing manganese ions by using an aluminum mesh-based lithium phosphate pole piece as a cathode, and nanometer lithium manganese phosphate is generated on the cathode.
[0038] The imidazole-based ionic liquid containing manganese ions used in this example is an organic molten salt in a liquid state at or near room temperature. Compared with traditional organic solvents, it is completely composed of anions and cations, and the relatively high ionic environme...
Embodiment 2
[0056] In this embodiment, step S1 includes the following sub-steps:
[0057] S1.1, 100mL of LiOH·H with a concentration of 1mol / L 2 O solution was stirred and heated to 50°C and kept constant;
[0058] S1.2, using a peristaltic pump in LiOH·H 2 Add 50 mL of H with a concentration of 0.6 mol / L dropwise into the O solution 3 PO 4 Solution, the dropping rate is 5mL / min;
[0059] S1.3. Leave the solution formed in step S1.2 to stand, and the chemical reaction 3LiOH+H occurs 3 PO 4 → Li 3 PO 4 (S)+3H 2 O gives the precipitated product solid Li 3 PO 4 ;
[0060] S1.4. Wash the precipitated product, dry it in vacuum, and then calcinate at 300°C for 4h to form white Li 3 PO 4 Powder;
[0061] S1.5, the aluminum mesh and the white Li 3 PO 4 The powder is pressed into an aluminum mesh-based lithium phosphate pole piece by a tablet machine, wherein, during the pressing process, the pressure is 10 MPa, and the pressure is maintained for 2 minutes. The aluminum mesh base ...
Embodiment 3
[0071] Specifically, step S1 includes the following sub-steps:
[0072] S1.1, 100mL of LiOH·H with a concentration of 1mol / L 2 O solution was stirred and heated to 50°C and kept constant;
[0073] S1.2, using a peristaltic pump in LiOH·H 2 Add 50 mL of H with a concentration of 0.6 mol / L dropwise into the O solution 3 PO 4 Solution, the dropping rate is 5mL / min;
[0074] S1.3. Leave the solution formed in step S1.2 to stand, and the chemical reaction 3LiOH+H occurs 3 PO 4 → Li 3 PO 4 (S)+3H 2 O, to obtain the precipitated product solid Li 3 PO 4 ;
[0075] S1.4. Wash the precipitated product, dry it in vacuum, and then calcinate at 300°C for 4h to form white Li 3 PO 4 Powder;
[0076] S1.5, the aluminum mesh and the white Li 3 PO 4 The powder is pressed into an aluminum mesh-based lithium phosphate pole piece by a tablet machine, wherein, during the pressing process, the pressure is 10 MPa, and the pressure is maintained for 2 minutes. The aluminum grid base l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com