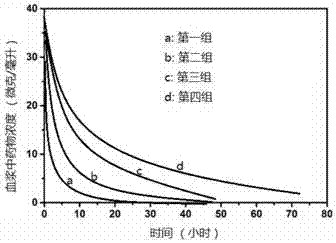
Dihydroartemisinin diploid derivative, and medicine composition and application thereof
A technology of dihydroartemisinin and derivatives, applied in the field of medicine, can solve the problems of no diploid amphiphilic molecules of artemisinin, poor water solubility of artemisinin, dihydroartemisinin, and artesunate , to achieve a good effect of killing malaria parasites and a good anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
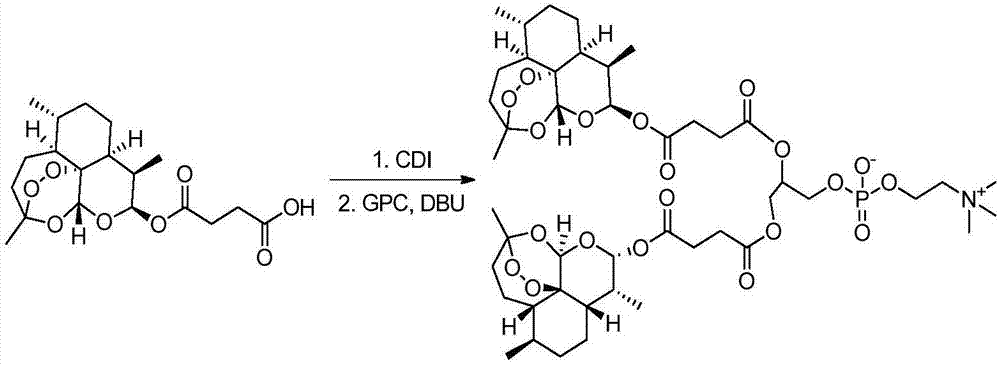
[0131] Synthesis of dihydroartemisinin diploid phosphatidylcholine (see the synthetic route figure 2 )
[0132] Dissolve artesunate 0.128g, CDI 0.162g in 15mL dichloromethane, react at room temperature for 4 hours, spin dry to remove dichloromethane, dissolve the residue in dimethyl sulfoxide 10mL, add glycerophosphorylcholine 0.026g, DBU 0.05 g, react overnight at 40°C. The product is purified with a flash preparative chromatography system (chromatographic column: silica gel, eluent: dichloromethane / methanol), and the product is a white solid with a yield of 35%. The purity is 97.3% as detected by high performance liquid chromatography, and the solubility in water at 20°C It is 16.96g / L (artesunate is only 0.36g / L). Mass Spectrum (MS) (m / z): [M+Na] + 1012.42. 1 H-NMR (500MHz, DMSO-d 6 )See image 3 : δ5.67(d,J=8.3Hz,2H,H-10,10'),5.55(s,2H,H-12,12'),4.29–4.10(m,1H,H-20),4.03 -3.75(m,4H,H-21,22),3.51-3.47(m,4H,H-23,24),3.13(s,9H,H-25,26,27),2.72-2.54(m, 8H,H-18,18',17,...
Embodiment 2
[0134] Synthesis of dihydroartemisinin dithiodiacetic acid diploid phosphatidylcholine (see the synthetic route image 3 )
[0135] 1.5 g of dihydroartemisinin and 1 g of dithiodiacetic anhydride were dissolved in 20 mL of chloroform, 0.5 g of triethylamine was added, and the reaction was stirred overnight at 40°C. The solvent was removed, and the flash preparative chromatography system was used for separation and purification (chromatographic column: silica gel, eluent: dichloromethane / methanol), and the product dihydroartemisinin dithiodiacetic acid was obtained as a white solid 1.8g.
[0136] Dihydroartemisinin dithiodiacetic acid is 0.5g of white solid, 0.3g of CDI is dissolved in 15mL of dichloromethane, reacted at room temperature for 3 hours, spin-dried to remove dichloromethane, the residue is dissolved in 10mL of dimethyl sulfoxide, Add 0.12 g of glycerophosphorylcholine and 0.3 g of DBU, and react overnight at 40°C. The product was purified with a flash preparative...
Embodiment 3
[0139] Synthesis of dihydroartemisinin dithiodiacetic acid diploid carboxybetaine (see the synthetic route Figure 4 )
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com