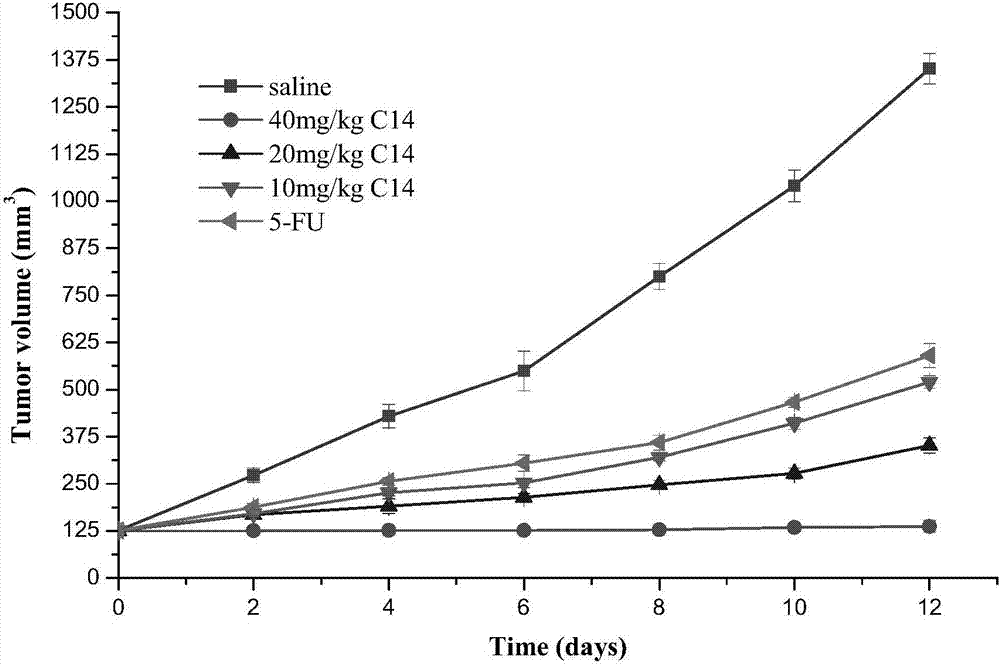
Tumor microtubulin HIF-1alpha double target inhibitor and preparation method thereof
A technology of tubulin and HIF-1, which is applied in the field of malignant tumors and can solve problems such as poor efficacy of blood vessel blocking agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] Such as figure 1 As shown, the preparation method of the tumor tubulin HIF-1α dual-target inhibitor provided in the embodiment of the present invention includes the following steps:
[0022] S101: Add 3', 4', 5'-trimethoxy-7-hydroxyflavone or 2', 3', 4'-trimethoxy-7-hydroxyflavone or 4', 5 ', 6'-trimethoxy-7-hydroxyflavone (3.31g, 0.01mol), anhydrous potassium carbonate (5.52g, 0.04mol) and acetone (100ml), heated and stirred to reflux, then added dropwise 1,2- Dibromoethane or 1,3-dibromopropane or 1,4-dibromobutane (0.04mol) was heated and condensed to reflux at 60°C, the solution became clear and then cloudy. TCL (thin-layer chromatography) detects the reaction process, and column chromatography purifies, and the eluent is: methanol: dichloromethane=1:50;
[0023] S102: add benzimidazole derivative (0.015mol) in 250mL there-necked flask, make it dissolve in 100mL acetone, add potassium carbonate (5.52g, 0.04mol), after stirring for 10min, add the flavone derivative...
Embodiment 1
[0027] Example 1: 7-(2-bromoethoxy)-2-(3,4,5-trimethylphenyl)-4H-benzopyran-4-one
[0028] In a 250mL round bottom flask, add 3', 4', 5'-trimethoxy-7-hydroxyflavone (3.31g, 0.01mol), anhydrous potassium carbonate (5.52g, 0.04mol) and acetone (30ml) successively, Heat and stir to reflux, then add 1,2-dibromoethane (0.04mol) dropwise, heat and condense to reflux at 60°C, the solution becomes clear and then turbid. TCL (thin-layer chromatography) detects the reaction process, and purifies by column chromatography, the eluent is: methanol:dichloromethane=1:50. Yellow powder, the yield is 80%. 1 H NMR (400MHz, cdcl 3 )δ7.72(d, J=8.5Hz, 1H), 7.14(s, 2H), 6.78(d, J=2.1Hz, 1H), 6.76(d, J=2.2Hz, 1H), 6.75–6.73( m, 2H), 4.40 (t, J = 6.2Hz, 2H), 3.92 (d, J = 10.7Hz, 9H), 3.68 (t, J = 6.2Hz, 2H).
[0029]
Embodiment 2
[0030] Example 2: 7-(2-(1H-benzo[d]imidazol-1-yl)ethoxy)-2-(3,4,5-trimethoxyphenyl)-4H-benzopyran -4-one
[0031] Add benzimidazole (0.0015mol) in 100mL three-necked flask, make it dissolve in 30mL acetone, add potassium carbonate (0.552g, 0.004mol), after stirring for 10min, add the pale yellow solid 7-(2- Bromoethoxy)-2-(3,4,5-trimethylphenyl)-4H-benzopyran-4-one (0.001mol), tetrabutylammonium bromide (TBAB) (0.03g, 0.0001mol), heated to 60°C and stirred for reaction, (TCL detection reaction process), extracted with ethyl acetate, washed the organic layer with saturated brine, then dried with anhydrous sodium sulfate, evaporated the solvent to dryness under reduced pressure and separated it with a silica gel column. The target compound was obtained, and the eluent was: methanol:dichloromethane=1:50. Yellow powder, yield 51%. 1 H NMR (400MHz, cdcl 3 )δ8.03(s, 1H), 7.82(d, J=7.0Hz, 1H), 7.68(d, J=8.6Hz, 1H), 7.47(d, J=7.3Hz, 1H), 7.32(dq, J=7.2, 6.0Hz, 2H), 7.11(s, 2H), 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com