Preparation method of uracil
A technology of uracil and ethyl acetate, which is applied in the field of uracil preparation, can solve the problems of limited application range, expensive raw materials and catalysts, etc., and achieve the effects of simple and fast operation, low production cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
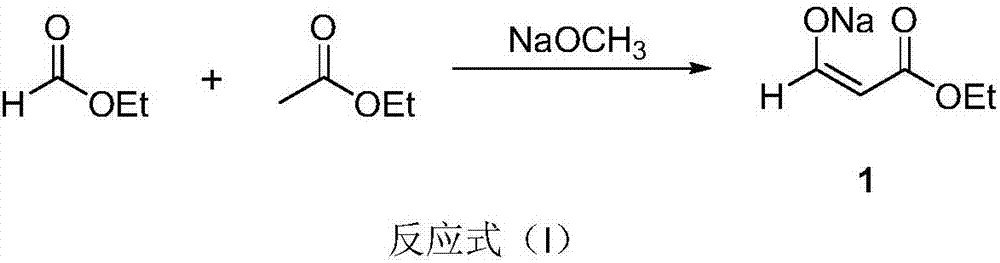
[0021] Add sodium methoxide (4.32g, 0.08mol) and ethyl acetate (0.13mol, 12.5mL) into a 50mL reaction flask, stir at room temperature for 10min, slowly add ethyl formate (0.05mol, 4.1mL) dropwise, and complete the dropwise addition in 30min . Transfer to a 35°C oil bath to continue the reaction. The system dissolves first, and a large amount of white solids are produced after a period of time. After 4 hours, GC-MS detected that the ethyl formate was completely reacted, and it was directly used in the next reaction without separation.
Embodiment 2
[0023] Weigh thiourea (0.045mol, 3.42g), grind it and add it to the crude product obtained in Example 1, the reaction solution becomes viscous, add 15mL of ethyl acetate to dilute the reaction system, stir and react at 60°C for 2 hours, TLC detects the reaction Finish. Concentrate the reaction solution to dryness, add 40 mL of hydrochloric acid solution (3mol / L) to the obtained white solid to make a slurry for 1 hour, then suction filter, rinse the filter cake with 10 mL of water, and dry it in vacuum at 50°C for 4 hours to obtain 4.25 g of white solid powder, two The combined yield is 74%, and the purity is 98.97%.
[0024] 1 H NMR (400MHz, DMSO) δ12.43(s, 1H), 12.26(s, 1H), 7.41(d, J=7.5Hz, 1H), 5.82(d, J=7.6Hz, 1H).
Embodiment 3
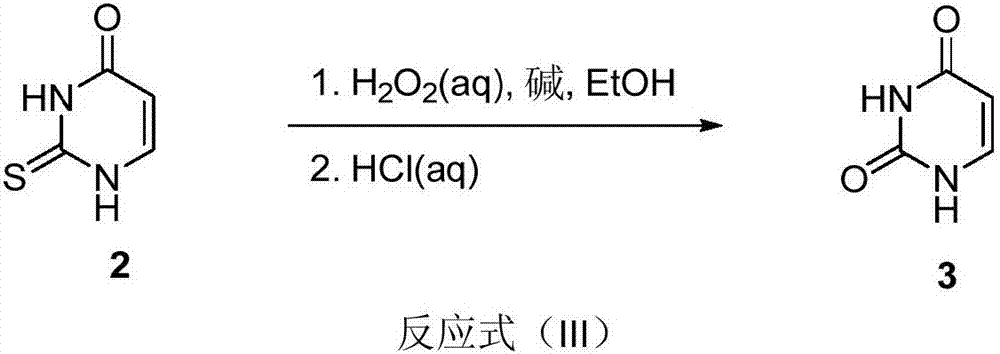
[0026] Add thiouracil (4.15g, 0.033mol), potassium hydroxide (2.79g, 0.05mol) and 30mL ethanol to a 100mL reaction flask, cool to 0‐5°C, slowly add 30% Wt H2O2 (10.2ml, 0.1 mol), after the dropwise addition was completed, it was transferred to an oil bath at 60°C and stirred for 4 hours. After the reaction was detected by TLC, it was cooled to room temperature and filtered with suction, and the filter cake was washed with 5 mL of ethanol. Add 15mL of water to the obtained off-white solid and stir evenly, then slowly add 4.5mL of concentrated hydrochloric acid dropwise, transfer to an oil bath at 60°C and stir for 1 hour after the dropwise addition is completed, after the system is cooled to room temperature, suction filter, and the filter cake is washed with 5mL of cold water , the obtained white powder was dried in vacuum at 50° C. for 5 hours to obtain 2.37 g of white powdery solid, yield: 65.3%, purity 99%.
[0027] 1 H NMR (400 MHz, DMSO) δ 10.97 (s, 1H), 10.78 (s, 1H), 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com