2,4,6-tri(dimethyl phosphate amido)-1,3,5-triazine-tri-melamine salt and preparation method and application thereof
A technology based on dimethyl phosphate amine group and diformyl chloride phosphate amine group, which is applied in the field of organic compound synthesis and can solve the problems of limited species
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
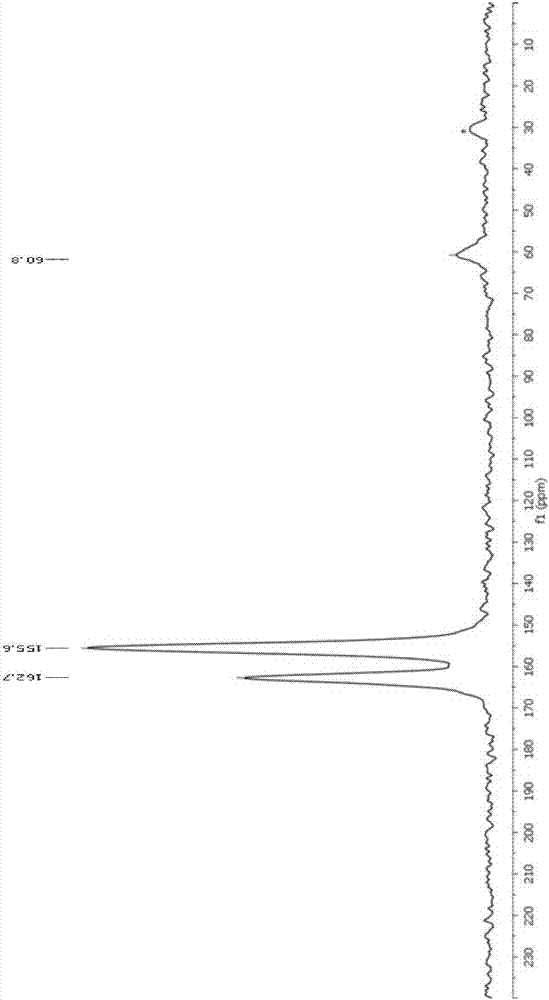
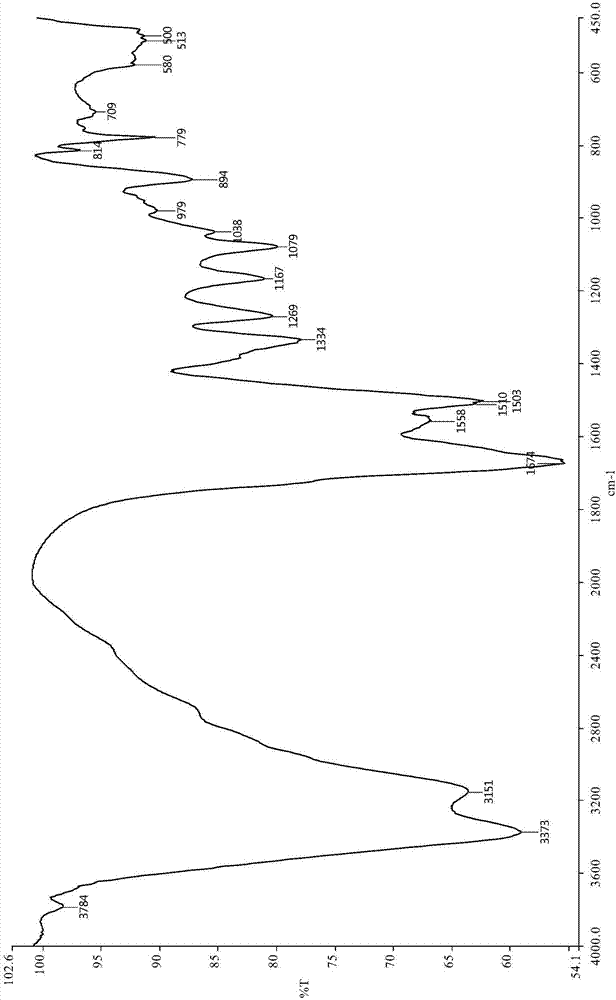
Image
Examples
preparation example Construction
[0034] The present invention provides a kind of preparation method of described 2,4,6-tris(dimethyl phosphate amino group)-1,3,5-triazine-tri-melamine salt, comprising the following steps:
[0035] Carrying out primary heat treatment to the mixture of hexamethylolmelamine and phosphorus oxychloride, and performing secondary heat treatment after heating up to obtain a secondary treatment product;
[0036] The secondary treatment product is dissolved in an inert solvent, followed by atmospheric distillation and vacuum distillation to obtain 2,4,6-tris(phosphoric acid diformyl chloride amino)-1,3,5-triazine solution; the inert solvent is 1,2-dichloroethane, 1,1,1-trichloroethane, 1,1,2,2-tetrachloroethane, chlorobenzene, nitrobenzene, dimethyl ethylene One or more of sulfone, sulfolane and N,N-dimethylformamide;
[0037] The 2,4,6-tris(phosphoric acid diformyl chloride amino group)-1,3,5-triazine and melamine are hydrolyzed into a salt reaction in water to obtain 2,4,6-tris(dime...
Embodiment 1
[0057] 10g of hexamethylolmelamine and 60.08g of phosphorus oxychloride (molar ratio 1:12) were stirred and reacted in a 150ml three-necked flask. The feeding process is to first add phosphorus oxychloride in an oil bath at 45°C. After the temperature is stable, add hexamethylolmelamine in 12 times, about 0.8g each time. After each addition, wait until the liquid in the bottle is clear and the temperature drops to 45°C. For the next addition, the temperature was maintained at 45°C, and the total addition time was two hours. After the addition, the temperature was kept at 50° C., and the reaction was carried out for 1 hour. Then, raise the temperature to 80°C (heating time is half an hour) and keep it for 5 hours. At this time, the bottle is a colorless transparent liquid, that is, 2,4,6-tris(phosphoric acid diformyl chloride amino)-1,3, 5-triazine.
[0058] The obtained 2,4,6-tris(phosphodiformylchloroamino)-1,3,5-triazine was dissolved in dimethyl sulfoxide. Then change to...
Embodiment 2
[0064] Hexamethylolmelamine and phosphorus oxychloride (molar ratio 1:3) were stirred and reacted in a 150ml three-necked flask. The feeding process is to first add phosphorus oxychloride in an oil bath at 45°C, and then add hexamethylolmelamine in 12 times after the temperature is stable. At 50°C, the total addition time was two hours. After the addition was complete, the temperature was kept at 45° C., and the reaction was carried out for 1 hour. Then, raise the temperature to 75°C (heating time is half an hour) and keep it for 5 hours. At this time, the bottle is a colorless transparent liquid, that is, 2,4,6-tris(phosphoric acid diformyl chloride amino)-1,3, 5-triazine.
[0065]The obtained 2,4,6-tris(phosphodiformylchloroamino)-1,3,5-triazine was dissolved in 1,1,1-trichloroethane. Then change to a distillation device, 125 ℃ oil bath distillation (heating up for half an hour, distillation for half an hour), until basically no fractions are distilled out, and then coole...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com