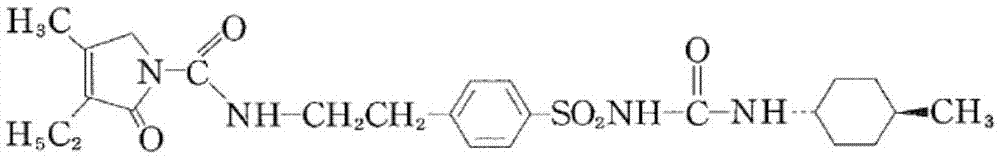
Glimepiride tablet and preparation method thereof
A technology for glimepiride tablets and urea tablets, applied in the field of medicine, can solve the problems of large differences in dissolution between tablets, low bioavailability, low solubility of insoluble drugs, etc., and achieves rapid drug dissolution and simple preparation process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] Preparation Process:
[0029] Dissolve glimepiride in sodium hydroxide solution, add mesoporous silica, stir evenly, add glacial acetic acid to the solution under stirring condition, the compound of glimepiride and mesoporous silica is precipitated, Filter, dry at 60°C, then mix with microcrystalline cellulose and sodium carboxymethyl starch, mix evenly, add magnesium stearate, mix, and compress into tablets.
Embodiment 2
[0031]
[0032] Preparation Process:
[0033] Dissolve glimepiride in sodium hydroxide solution, add mesoporous silica, stir evenly, add glacial acetic acid to the solution under stirring condition, the compound of glimepiride and mesoporous silica is precipitated, Filtrate, dry at 65°C, then mix evenly with the mixed powder of microcrystalline cellulose and crospovidone, add magnesium stearate, mix, and compress into tablets.
Embodiment 3
[0035]
[0036] Preparation Process:
[0037] Dissolve glimepiride in sodium hydroxide solution, add mesoporous silica, stir evenly, add glacial acetic acid to the solution under stirring condition, the compound of glimepiride and mesoporous silica is precipitated, Filter, dry at 60°C, then mix with microcrystalline cellulose and sodium carboxymethyl starch, mix evenly, add magnesium stearate, mix, and compress into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com