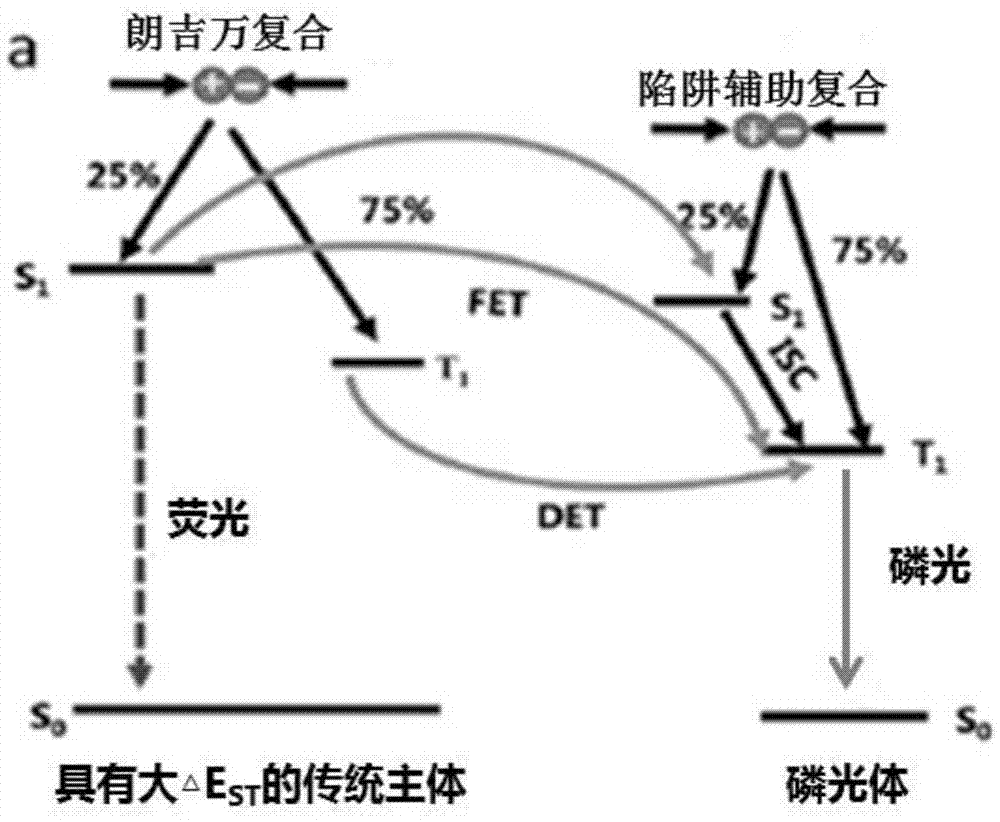
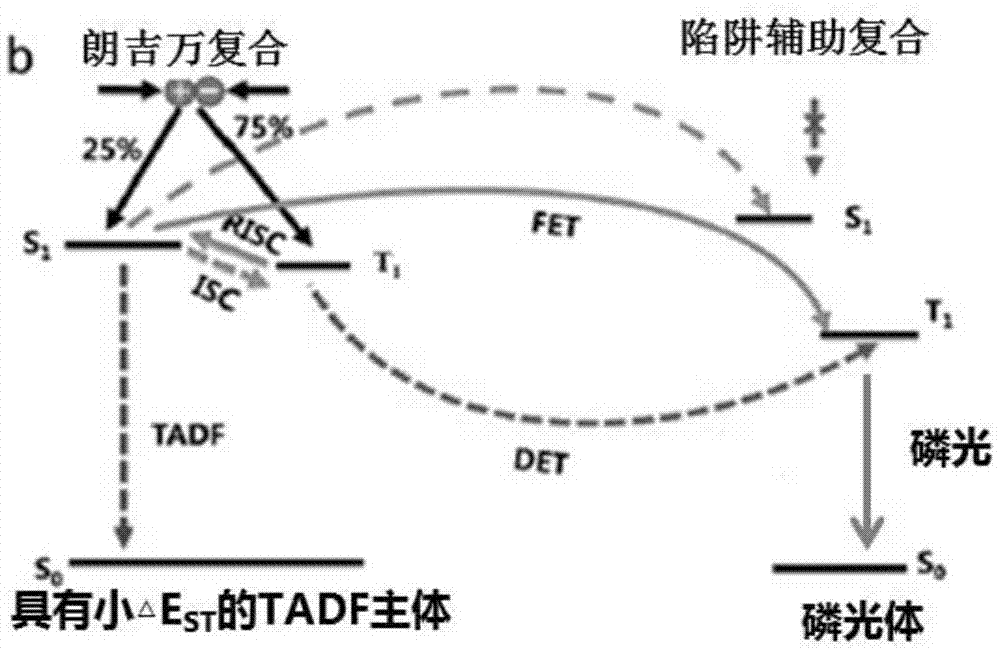
Red phosphorescent organic electroluminescent device
A red phosphorescence and electroluminescence technology, applied in electric solid devices, electrical components, semiconductor devices, etc., can solve the problem of low oxidation stability of host materials, improve exciton utilization, reduce triplet-triplet annihilation, The effect of reducing the driving voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Synthesis of compounds of structure shown in formula (1-1): under nitrogen range, 3,6-dibromo-9-fluorenone (5mmol), phenoxazine (18mmol), Pd 2 (dba) 3 (0.8mmol), NaOtBu (30mmol) and tBu 3 P·HBF 4 (0.8 mmol) was put into 100 mL of toluene and stirred overnight at a temperature of 105°C. The reaction was quenched by adding 10 mL of cold water to the mixture. After the mixture was cooled to room temperature, it was vacuum filtered, followed by purification by column chromatography to obtain the product with the structure shown in formula (1-1), and the product was dried in vacuum. Yield: 80%.
[0067] The molecular weight obtained by mass spectrometry: 542.58.
[0068] The relative molecular mass percentages of each element obtained by elemental analysis: C: 81.90%; H: 4.09%; N: 5.16%; O: 8.85%.
Embodiment 2
[0070] Synthetic compound of structure shown in formula (1-2): reactant phenoxazine is replaced by phenothiazine, through the same synthetic method as Synthetic Example 1, the compound of structure shown in formula (1-2) is obtained, productive rate 91% .
[0071] The molecular weight obtained by mass spectrometry: 574.71.
[0072] The relative molecular mass percentages of each element obtained by elemental analysis: C: 77.32%; H: 3.86%; N: 4.87%; O: 2.78%; S, 11.16%.
Embodiment 3
[0074] Synthetic compound of structure shown in formula (1-3): the reactant phenoxazine is replaced by 9,9-dimethylacridine, through the same synthetic method as in Synthesis Example 1, the structure shown in formula (1-3) is obtained Compound, yield 87%.
[0075] The molecular weight obtained by mass spectrometry: 594.74.
[0076] The relative molecular mass percentages of each element obtained by elemental analysis: C: 86.84%; H: 5.76%; N: 4.71%; O: 2.69%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com