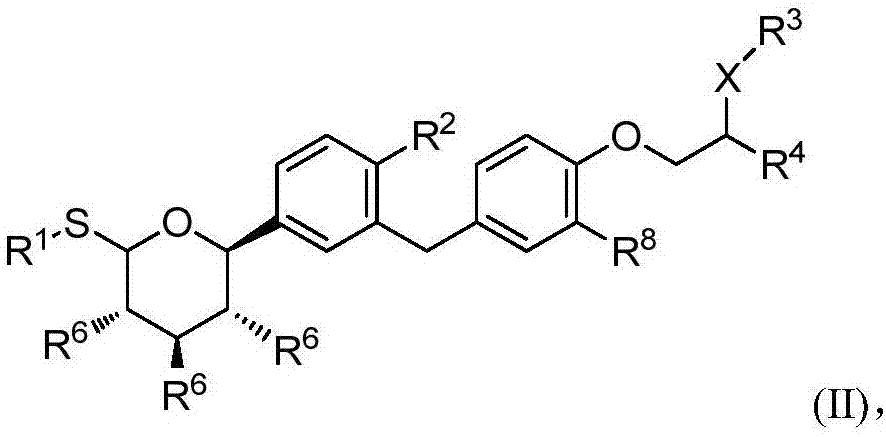
Glucopyranosyl derivative and application thereof in medicine
An alkyl and hydroxyl technology, applied in the field of glucopyranosyl derivatives, can solve the problems of undiscovered and low risk of hypoglycemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0183] Example 1 (2S, 3R, 4R, 5S, 6R)-2-[4-chloro-3-[[4-[[(2S)-oxirane-2-yl]methoxy]phenyl] Methyl]phenyl]-6-methylthiotetrahydropyran-3,4,5-triol 1
[0184]
[0185]
[0186] Step 1 (3aS, 3bR, 7aS, 8aS)-2,2,5,5-Tetramethyltetrahydro-3aH-[1,3]dioxolano[4′,5′: 4, 5] Furo[3,2-d][1,3]dioxane 1b
[0187] Magnesium sulfate (160g, 1.33mol) and concentrated sulfuric acid (9.9mL, 190mmol) were added to (2S,3R,4S)-2,3,4,5-tetrahydroxypentanal 1a (99.0g, 659mmol) at room temperature In acetone (1.2 L) solution, the resulting reaction system was stirred for 12 hours. The reaction mixture was suction-filtered, washed with acetone (300mL×2), the filtrates were combined, and the pH value was adjusted to 7 with 25% ammonia water. Solids were precipitated, then suction-filtered again, and washed with acetone (150mL×2). The filtrates were combined and concentrated under reduced pressure to obtain the title compound 1b as a yellow oil (128.0 g, 84.3%).
[0188] 1 H NMR (600MHz, CDCl ...
Embodiment 2
[0231] Example 2 (2S, 3R, 4R, 5S, 6R)-2-[4-chloro-3-[[4-[(2S)-3-ethoxy-2-hydroxypropoxy]phenyl]methanol Base]phenyl]-6-methylthiotetrahydropyran-3,4,5-triol 2
[0232]
[0233]
[0234] At room temperature, chopped metallic sodium (195 mg, 8.48 mmol) was added into absolute ethanol (20 mL), and stirred until the sodium block completely disappeared. (2S, 3R, 4R, 5S, 6R)-2-[4-chloro-3-[[4-[[(2S)-oxirane-2-yl]methoxy]phenyl]methyl ]Phenyl]-6-methylthiotetrahydropyran-3,4,5-triol 1 (0.48g, 1.1mmol) in ethanol (10mL) was added to the above reaction solution, heated to 60°C, and stirred for reaction 6 hours. Add glacial acetic acid (1mL) to quench, concentrate under reduced pressure to remove the solvent, add ethyl acetate (20mL) and saturated aqueous sodium bicarbonate solution (20mL) to the residue, let the layers stand, and wash the organic phase with saturated brine (10mL× 3), concentrated under reduced pressure, and the residue was purified by silica gel column chromat...
Embodiment 3
[0237]Example 3 (2S, 3R, 4R, 5S, 6R)-2-[4-chloro-3-[[4-[[(2R)-oxirane-2-yl]methoxyl]phenyl] Methyl]phenyl]-6-methylthiotetrahydropyran-3,4,5-triol 3
[0238]
[0239] At room temperature, the compound (2S, 3R, 4R, 5S, 6R)-2-[4-chloro-3-[(4-alkylphenyl)methyl]phenyl]-6-methylthiotetrahydropyridine Furan-3,4,5-triol 1o (see step 13 of Example 1, 58 mg, 0.15 mmol) was dissolved in ethanol (2 mL), and (2S)-2-(chloromethyl)oxirane (0.4 mL, 5mmol) and potassium carbonate (15mg, 0.11mmol), the temperature was raised to 73°C, and the reaction was stirred for 5 hours. After filtration, the filtrate was concentrated under reduced pressure, and the residue was purified by silica gel column chromatography [100% ethyl acetate] to obtain the title compound 3 as a white solid (12 mg, 16.4%, HPLC: 90.5%).
[0240] MS (ESI, pos.ion) m / z: 475.2 [M+Na] + ;
[0241] 1 H NMR (600MHz, CD 3 OD) δ (ppm): 7.38 (d, 1H), 7.26 (d, 2H), 7.14 (d, 2H), 6.88 (d, 2H), 4.40 (d, 1H), 4.29 (dd, 1H), 4.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com